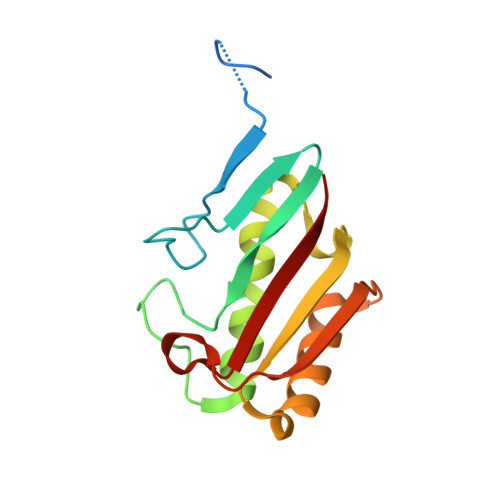
Crystal structures of the putative endoribonuclease L-PSP from Entamoeba histolytica.
Ojuromi, O.T., Giwa, A.O., Gardberg, A., Subramanian, S., Myler, P.J., Abendroth, J., Staker, B., Asojo, O.A.(2025) Acta Crystallogr F Struct Biol Commun 81: 226-234
- PubMed: 40314238
- DOI: https://doi.org/10.1107/S2053230X25003875
- Primary Citation of Related Structures:
3M1X, 3M4S, 3MQW - PubMed Abstract:
Entamoeba histolytica causes amebiasis, a neglected disease that kills ∼100 000 people globally each year. Due to emerging drug resistance, E. histolytica is one of the target organisms for structure-based drug discovery by the Seattle Structural Genomics Center for Infectious Disease (SSGCID). Purification, crystallization and three structures of the putative drug target endoribonuclease L-PSP from E. histolytica (EhL-PSP) are presented. EhL-PSP has a two-layer α/β-sandwich with structural homology to endoribonuclease L-PSP. All three structures reveal the prototypical YjgF/YER057c/UK114 family trimer topology with accessible allosteric active sites. Citrate molecules from the crystallization solution are bound to the allosteric site in two of the three reported structures. The large allosteric site of EhL-PSP is well conserved with bacterial YjgF/YER057c/UK114 family members and could be targeted for inhibition, drug discovery or repurposing.
- Zoology and Environmental Biology, Lagos State University, Nigeria.
Organizational Affiliation: