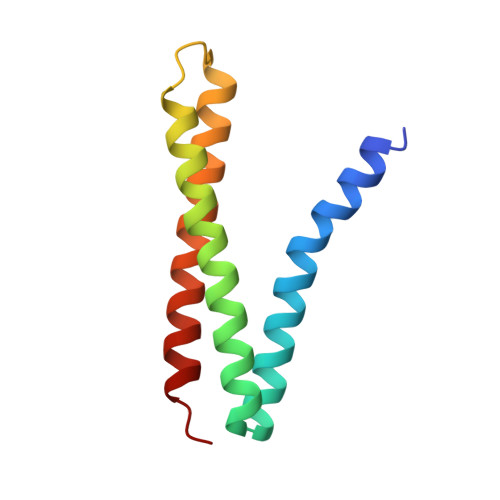
Der p 5 crystal structure provides insight into the group 5 dust mite allergens.
Mueller, G.A., Gosavi, R.A., Krahn, J.M., Edwards, L.L., Cuneo, M.J., Glesner, J., Pomes, A., Chapman, M.D., London, R.E., Pedersen, L.C.(2010) J Biological Chem 285: 25394-25401
- PubMed: 20534590
- DOI: https://doi.org/10.1074/jbc.M110.128306
- Primary Citation of Related Structures:
3MQ1 - PubMed Abstract:
Group 5 allergens from house dust mites elicit strong IgE antibody binding in mite-allergic patients. The structure of Der p 5 was determined by x-ray crystallography to better understand the IgE epitopes, to investigate the biologic function in mites, and to compare with the conflicting published Blo t 5 structures, designated 2JMH and 2JRK in the Protein Data Bank. Der p 5 is a three-helical bundle similar to Blo t 5, but the interactions of the helices are more similar to 2JMH than 2JRK. The crystallographic asymmetric unit contains three dimers of Der p 5 that are not exactly alike. Solution scattering techniques were used to assess the multimeric state of Der p 5 in vitro and showed that the predominant state was monomeric, similar to Blo t 5, but larger multimeric species are also present. In the crystal, the formation of the Der p 5 dimer creates a large hydrophobic cavity of approximately 3000 A(3) that could be a ligand-binding site. Many allergens are known to bind hydrophobic ligands, which are thought to stimulate the innate immune system and have adjuvant-like effects on IgE-mediated inflammatory responses.
- Laboratory of Structural Biology, National Institutes of Health, Research Triangle Park, North Carolina 27709, USA. mueller3@niehs.nih.gov
Organizational Affiliation: