Crystal Structure of a Non-Neutralizing HIV-1 gp41 Envelope Antibody Demonstrates Neutralization Mechanism of gp41 Antibodies
Nicely, N.I., Dennison, S.M., Kelsoe, G., Ueda, Y., Liao, H.-X., Alam, S.M., Haynes, B.F.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Find similar proteins by: Sequence | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| gp41 MPER-derived peptide | A [auth P] | 18 | N/A | Mutation(s): 0 | 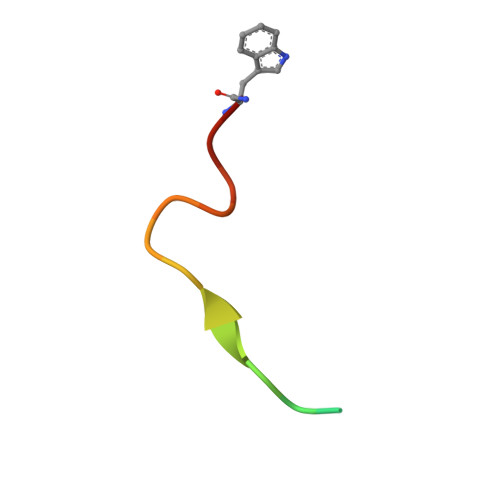 |
UniProt | |||||
Find proteins for P04580 (Human immunodeficiency virus type 1 group M subtype D (isolate Z6)) Explore P04580 Go to UniProtKB: P04580 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P04580 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ANTI-HIV-1 ANTIBODY 2F5 LIGHT CHAIN | B [auth L] | 214 | Homo sapiens | Mutation(s): 0 | 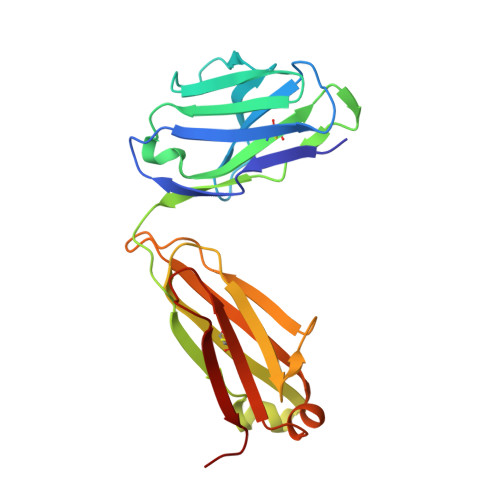 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ANTI-HIV-1 ANTIBODY 2F5 HEAVY CHAIN | C [auth H] | 237 | Homo sapiens | Mutation(s): 0 | 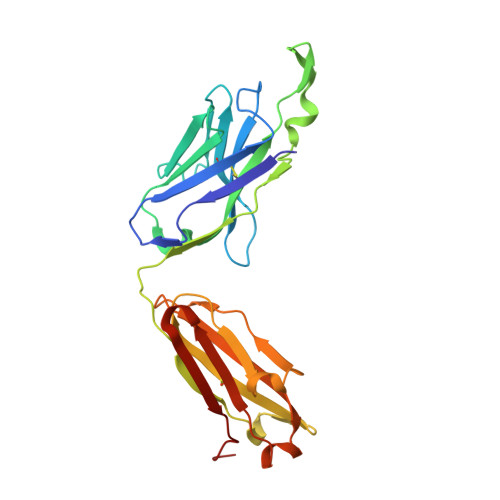 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 58.82 | α = 90 |
| b = 65.229 | β = 90 |
| c = 174.205 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | model building |
| PHENIX | refinement |
| d*TREK | data reduction |
| d*TREK | data scaling |
| PHENIX | phasing |