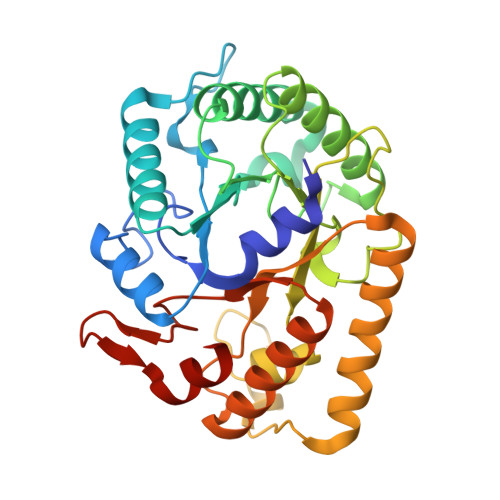
Biochemical characterization and crystal structure of endoglucanase Cel5A from the hyperthermophilic Thermotoga maritima.
Pereira, J.H., Chen, Z., McAndrew, R.P., Sapra, R., Chhabra, S.R., Sale, K.L., Simmons, B.A., Adams, P.D.(2010) J Struct Biol 172: 372-379
- PubMed: 20599513
- DOI: https://doi.org/10.1016/j.jsb.2010.06.018
- Primary Citation of Related Structures:
3MMU, 3MMW - PubMed Abstract:
Tm_Cel5A, which belongs to family 5 of the glycoside hydrolases, is an extremely stable enzyme among the endo-acting glycosidases present in the hyperthermophilic organism Thermotoga maritima. Members of GH5 family shows a common (β/α)(8) TIM-barrel fold in which the catalytic acid/base and nucleophile are located on strands β-4 and β-7 of the barrel fold. Thermally resistant cellulases are desirable for lignocellulosic biofuels production and the Tm_Cel5A is an excellent candidate for use in the degradation of polysaccharides present on biomass. This paper describes two Tm_Cel5A structures (crystal forms I and II) solved at 2.20 and 1.85Å resolution, respectively. Our analyses of the Tm_Cel5A structure and comparison to a mesophilic GH5 provides a basis for the thermostability associated with Tm_Cel5A. Furthermore, both crystal forms of Tm_Cel5A possess a cadmium (Cd(2+)) ion bound between the two catalytic residues. Activity assays of Tm_Cel5A confirmed a strong inhibition effect in the presence of Cd(2+) metal ions demonstrating competition with the natural substrate for the active site. Based on the structural information we have obtained for Tm_Cel5A, protein bioengineering can be used to potentially increase the thermostability of mesophilic cellulase enzymes.
- Physical Biosciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA.
Organizational Affiliation: