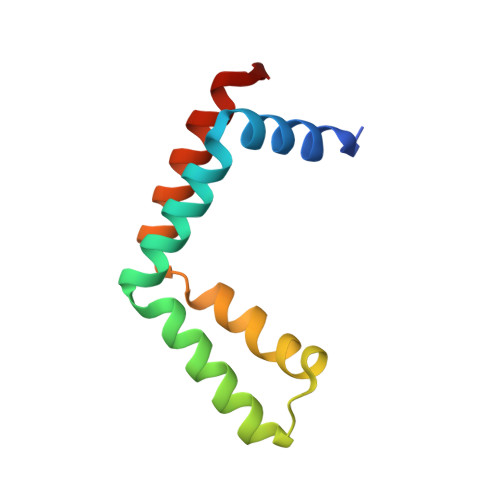
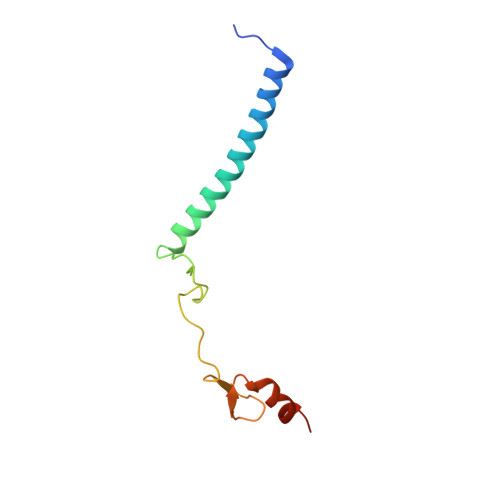
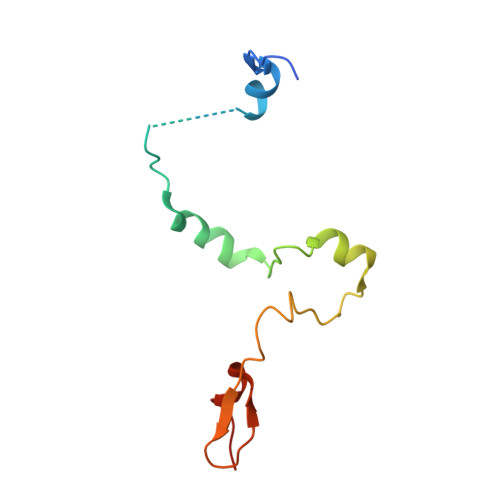
Structural insights into the assembly and function of the SAGA deubiquitinating module.
Samara, N.L., Datta, A.B., Berndsen, C.E., Zhang, X., Yao, T., Cohen, R.E., Wolberger, C.(2010) Science 328: 1025-1029
- PubMed: 20395473
- DOI: https://doi.org/10.1126/science.1190049
- Primary Citation of Related Structures:
3MHH, 3MHS - PubMed Abstract:
SAGA is a transcriptional coactivator complex that is conserved across eukaryotes and performs multiple functions during transcriptional activation and elongation. One role is deubiquitination of histone H2B, and this activity resides in a distinct subcomplex called the deubiquitinating module (DUBm), which contains the ubiquitin-specific protease Ubp8, bound to Sgf11, Sus1, and Sgf73. The deubiquitinating activity depends on the presence of all four DUBm proteins. We report here the 1.90 angstrom resolution crystal structure of the DUBm bound to ubiquitin aldehyde, as well as the 2.45 angstrom resolution structure of the uncomplexed DUBm. The structure reveals an arrangement of protein domains that gives rise to a highly interconnected complex, which is stabilized by eight structural zinc atoms that are critical for enzymatic activity. The structure suggests a model for how interactions with the other DUBm proteins activate Ubp8 and allows us to speculate about how the DUBm binds to monoubiquitinated histone H2B in nucleosomes.
- Department of Biophysics and Biophysical Chemistry, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Organizational Affiliation: