Axial Ligand Swapping In Double Mutant Maintains Intradiol-cleavage Chemistry in Protocatechuate 3,4-Dioxygenase
Purpero, V.M., Lipscomb, J.D.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protocatechuate 3,4-dioxygenase alpha chain | A, C [auth B], E [auth C] | 200 | Pseudomonas putida | Mutation(s): 0 Gene Names: pcaG EC: 1.13.11.3 | 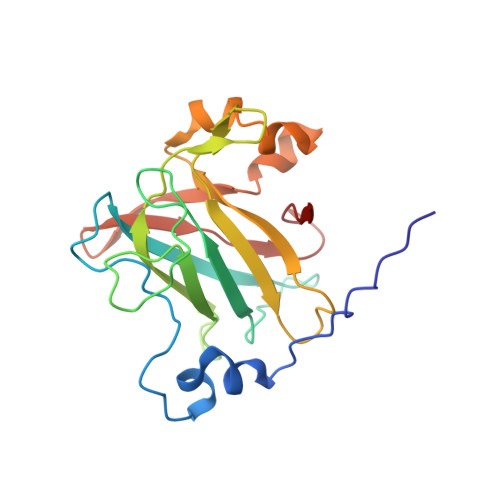 |
UniProt | |||||
Find proteins for P00436 (Pseudomonas putida) Explore P00436 Go to UniProtKB: P00436 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00436 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protocatechuate 3,4-dioxygenase beta chain | B [auth M], D [auth N], F [auth O] | 238 | Pseudomonas putida | Mutation(s): 2 Gene Names: pcaH EC: 1.13.11.3 | 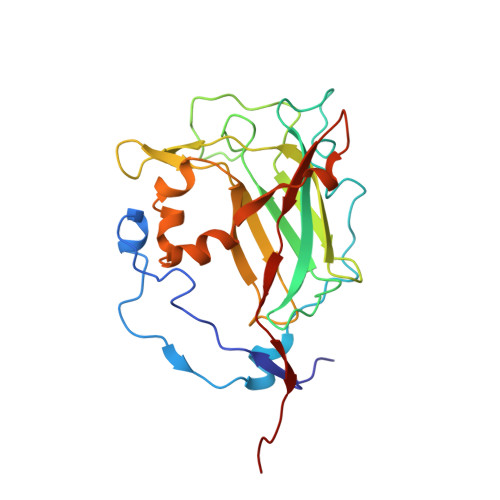 |
UniProt | |||||
Find proteins for P00437 (Pseudomonas putida) Explore P00437 Go to UniProtKB: P00437 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00437 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 7 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| DHY Query on DHY | AA [auth O], M, U [auth N] | 2-(3,4-DIHYDROXYPHENYL)ACETIC ACID C8 H8 O4 CFFZDZCDUFSOFZ-UHFFFAOYSA-N |  | ||
| SO4 Query on SO4 | G [auth A], N [auth B], S [auth N], V [auth C] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | H [auth A] I [auth A] O [auth B] T [auth N] W [auth C] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| BME Query on BME | J [auth A], P [auth B], Q [auth B] | BETA-MERCAPTOETHANOL C2 H6 O S DGVVWUTYPXICAM-UHFFFAOYSA-N |  | ||
| CO3 Query on CO3 | L [auth M] | CARBONATE ION C O3 BVKZGUZCCUSVTD-UHFFFAOYSA-L |  | ||
| FE Query on FE | K [auth M], R [auth N], X [auth O] | FE (III) ION Fe VTLYFUHAOXGGBS-UHFFFAOYSA-N |  | ||
| CL Query on CL | Z [auth O] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 128.319 | α = 90 |
| b = 140.769 | β = 90 |
| c = 168.183 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| MOLREP | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |