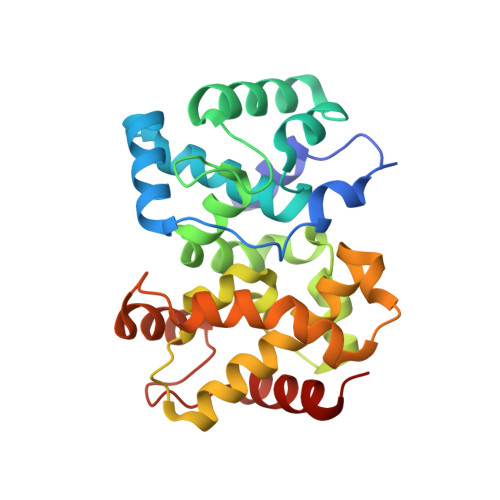
Structure of the Rift Valley fever virus nucleocapsid protein reveals another architecture for RNA encapsidation.
Raymond, D.D., Piper, M.E., Gerrard, S.R., Smith, J.L.(2010) Proc Natl Acad Sci U S A 107: 11769-11774
- PubMed: 20547879
- DOI: https://doi.org/10.1073/pnas.1001760107
- Primary Citation of Related Structures:
3LYF - PubMed Abstract:
Rift Valley fever virus (RVFV) is a negative-sense RNA virus (genus Phlebovirus, family Bunyaviridae) that infects livestock and humans and is endemic to sub-Saharan Africa. Like all negative-sense viruses, the segmented RNA genome of RVFV is encapsidated by a nucleocapsid protein (N). The 1.93-A crystal structure of RVFV N and electron micrographs of ribonucleoprotein (RNP) reveal an encapsidated genome of substantially different organization than in other negative-sense RNA virus families. The RNP polymer, viewed in electron micrographs of both virus RNP and RNP reconstituted from purified N with a defined RNA, has an extended structure without helical symmetry. N-RNA species of approximately 100-kDa apparent molecular weight and heterogeneous composition were obtained by exhaustive ribonuclease treatment of virus RNP, by recombinant expression of N, and by reconstitution from purified N and an RNA oligomer. RNA-free N, obtained by denaturation and refolding, has a novel all-helical fold that is compact and well ordered at both the N and C termini. Unlike N of other negative-sense RNA viruses, RVFV N has no positively charged surface cleft for RNA binding and no protruding termini or loops to stabilize a defined N-RNA oligomer or RNP helix. A potential protein interaction site was identified in a conserved hydrophobic pocket. The nonhelical appearance of phlebovirus RNP, the heterogeneous approximately 100-kDa N-RNA multimer, and the N fold differ substantially from the RNP and N of other negative-sense RNA virus families and provide valuable insights into the structure of the encapsidated phlebovirus genome.
- Life Sciences Institute, University of Michigan, Ann Arbor, MI 48109, USA.
Organizational Affiliation: