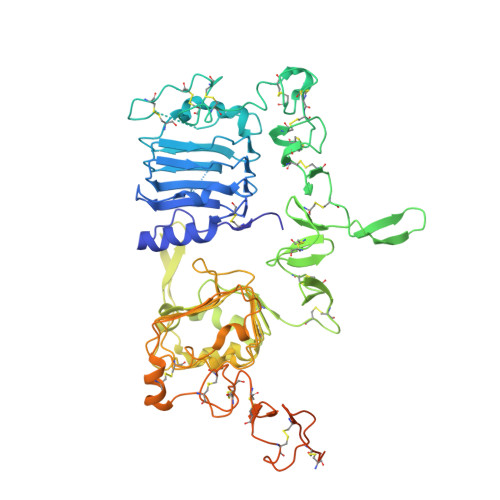
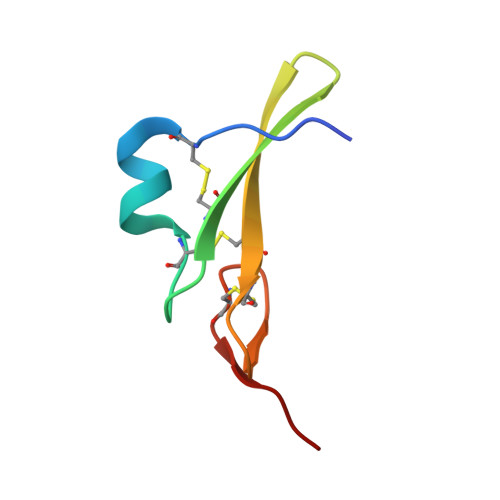
Structural basis for negative cooperativity in growth factor binding to an EGF receptor.
Alvarado, D., Klein, D.E., Lemmon, M.A.(2010) Cell 142: 568-579
- PubMed: 20723758
- DOI: https://doi.org/10.1016/j.cell.2010.07.015
- Primary Citation of Related Structures:
3LTF, 3LTG - PubMed Abstract:
Transmembrane signaling by the epidermal growth factor receptor (EGFR) involves ligand-induced dimerization and allosteric regulation of the intracellular tyrosine kinase domain. Crystallographic studies have shown how ligand binding induces dimerization of the EGFR extracellular region but cannot explain the "high-affinity" and "low-affinity" classes of cell-surface EGF-binding sites inferred from curved Scatchard plots. From a series of crystal structures of the Drosophila EGFR extracellular region, we show here how Scatchard plot curvature arises from negatively cooperative ligand binding. The first ligand-binding event induces formation of an asymmetric dimer with only one bound ligand. The unoccupied site in this dimer is structurally restrained, leading to reduced affinity for binding of the second ligand, and thus negative cooperativity. Our results explain the cell-surface binding characteristics of EGF receptors and suggest how individual EGFR ligands might stabilize distinct dimeric species with different signaling properties.
- Department of Biochemistry and Biophysics, University of Pennsylvania School of Medicine, Philadelphia, PA 19104-6059, USA.
Organizational Affiliation: