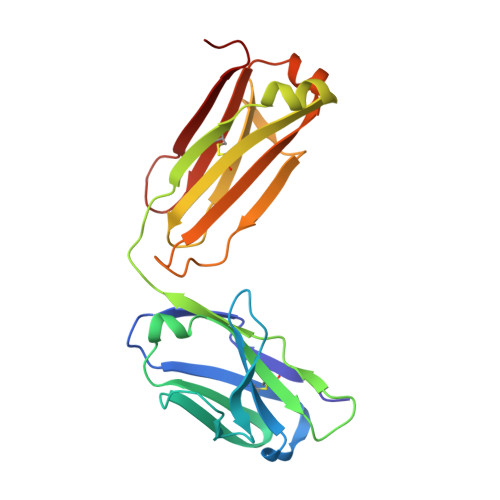
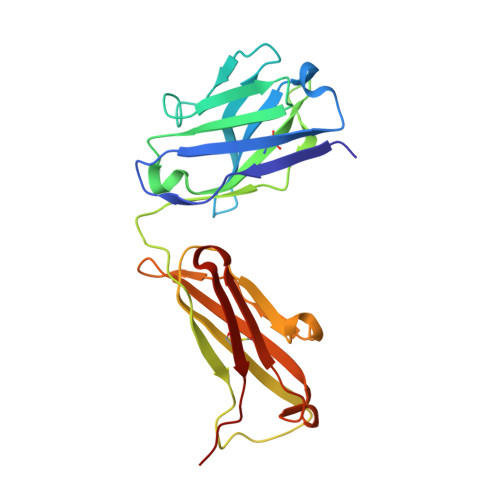
A structural insight into the molecular recognition of a (-)-Delta9-tetrahydrocannabinol and the development of a sensitive, one-step, homogeneous immunocomplex-based assay for its detection
Niemi, M.H., Turunen, L., Pulli, T., Nevanen, T.K., Hoyhtya, M., Soderlund, H., Rouvinen, J., Takkinen, K.(2010) J Mol Biology 400: 803-814
- PubMed: 20630472
- DOI: https://doi.org/10.1016/j.jmb.2010.05.048
- Primary Citation of Related Structures:
3LS4, 3LS5 - PubMed Abstract:
(-)-Delta9-tetrahydrocannabinol (THC) is the main psychoactive compound found in cannabis. In this study, an anti-THC Fab fragment, designed T3, was isolated from a display library cloned from the spleen cells of a mouse immunized with a THC-bovine serum albumin conjugate, and the crystal structures of the T3 Fab in its free form and in complex with THC were determined at 1.9 A and 2.0 A resolution, respectively. The THC binding site of the T3 Fab is a narrow cavity: the n-pentyl group of THC protrudes deep into the interface area between the variable domains and the C(10) monoterpene moiety of the hapten is partially exposed to solvent. The metabolites of THC, with modifications in the C(10) monoterpene moiety, 11-nor-9-carboxy-Delta(9)-tetrahydrocannabinol and 11-hydroxy-?(9)-tetrahydrocannabinol, are bound by the T3 Fab with a higher affinity than THC. The crystal structures suggest that Ser52H and Arg53H of the T3 Fab are able to make hydrogen bonds with the metabolites, which leads to an increased binding against these metabolites. By developing a T3 Fab-Delta(9)-THC immunocomplex binding antibody from a naïve antibody phage display library, the specificity of the Delta(9)-THC binding is highly increased, which allows a one-step, homogeneous, fluorescence resonance energy transfer-based sensitive immunoassay, with a detection limit of 20 ng/ml from saliva samples.
- Department of Chemistry, University of Eastern Finland, PO Box 111, FIN-80101 Joensuu, Finland.
Organizational Affiliation: