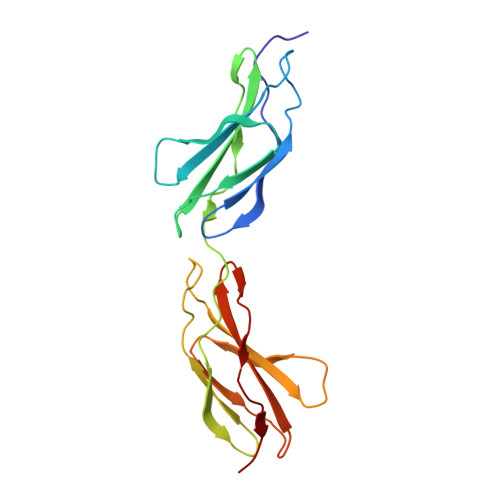
The structure of the FnIII Tandem A77-A78 points to a periodically conserved architecture in the myosin-binding region of titin
Bucher, R.M., Svergun, D.I., Muhle-Goll, C., Mayans, O.(2010) J Mol Biology 401: 843-853
- PubMed: 20542041
- DOI: https://doi.org/10.1016/j.jmb.2010.06.011
- Primary Citation of Related Structures:
3LPW - PubMed Abstract:
Titin is a large intrasarcomeric protein that, among its many roles in muscle, is thought to modulate the in vivo assembly of the myosin motor filament. This is achieved through the molecular template properties of its A-band region, which is composed of fibronectin type III (FnIII) and immunoglobulin (Ig) domains organized into characteristic 7-domain (D-zone) and 11-domain (C-zone) superrepeats. Currently, there is little knowledge on the structural details of this region of titin. Here we report the conformational characterization of three FnIII tandems, A77-A78, A80-A82, and A84-A86, which are components of the representative fourth C-zone superrepeat. The structure of A77-A78 has been elucidated by X-ray crystallography to 1.65 A resolution, while low-resolution models of A80-A82 and A84-A86 have been calculated using small-angle X-ray scattering. A77-A78 adopts an extended "up-down" domain arrangement, where domains are connected by a hydrophilic three-residue linker sequence. The linker is embedded in a rich network of polar contacts at the domain interface that results in a stiff molecular conformation. The models of A80-A82 and A84-A86, which contain hydrophobic six-residue-long interdomain linkers, equally showed elongated molecular shapes, but with slightly coiled or zigzagged conformations. Small-angle X-ray scattering data further suggested that the long linkers do not result in a noticeable increase in molecular flexibility but lead to semibent domain arrangements. Our findings indicate that the structural characteristics of FnIII tandems from A-band titin contrast markedly with those of poly-Ig tandems from the elastic I-band, which exhibit domain interfaces depleted of interactions and compliant conformations. Furthermore, the analysis of sequence conservation in FnIII domains from A-band titin points to the existence of conformationally defined interfaces at specific superrepeat positions, possibly leading to a periodic and locally ordered architecture supporting the molecular scaffold properties of this region of titin.
- Division of Structural Biology, Biozentrum, University of Basel, Klingelbergstr. 70, CH-4056 Basel, Switzerland.
Organizational Affiliation: