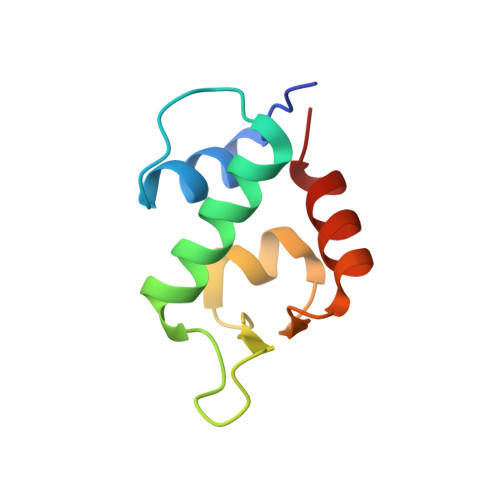
A left-handed solution to peptide inhibition of the p53-MDM2 interaction.
Liu, M., Pazgier, M., Li, C., Yuan, W., Li, C., Lu, W.(2010) Angew Chem Int Ed Engl 49: 3649-3652
- PubMed: 20449836
- DOI: https://doi.org/10.1002/anie.201000329
- Primary Citation of Related Structures:
3LNJ - PubMed Abstract:
[Image: see text] Throwing tumors a left hook punch: The oncoprotein MDM2 negatively regulates the activity and stability of the tumor suppressor protein p53, and is an important molecular target for anticancer therapy. Mirror image phage display identifies a high-affinity D-peptide ligand of MDM2 that can be developed into a potent and protease-resistant p53 activator with potential antitumor activity.
- Institute of Human Virology, University of Maryland School of Medicine, 725 W. Lombard St., Baltimore, MD 21201, USA.
Organizational Affiliation: