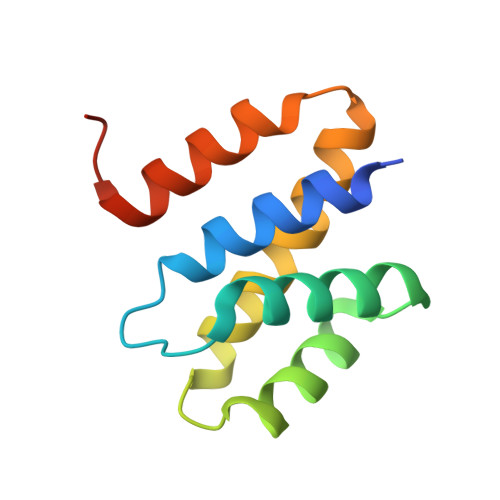
Structural basis for the function of the Saccharomyces cerevisiae Gfd1 protein in mRNA nuclear export.
Zheng, C., Fasken, M.B., Marshall, N.J., Brockmann, C., Rubinson, M.E., Wente, S.R., Corbett, A.H., Stewart, M.(2010) J Biological Chem 285: 20704-20715
- PubMed: 20463024
- DOI: https://doi.org/10.1074/jbc.M110.107276
- Primary Citation of Related Structures:
3LCN - PubMed Abstract:
Following transcription, mRNA is processed, packaged into messenger ribonucleoprotein (mRNP) particles, and transported through nuclear pores (NPCs) to the cytoplasm. At the NPC cytoplasmic face, Dbp5 mediates mRNP remodeling and mRNA export factor dissociation, releasing transcripts for translation. In Saccharomyces cerevisiae, the conserved poly(A) RNA-binding protein, Nab2, facilitates NPC targeting of transcripts and also modulates poly(A) tail length. Dbp5 removes Nab2 from mRNPs at the cytoplasmic face of the pore and, importantly, a Nab2 RNA-binding mutant suppresses the thermosensitive rat8-2 (dbp5) mutant. GFD1 is a multicopy suppressor of rat8-2 (dbp5), and Gfd1 interacts physically with both Dbp5 and the Nab2 N-terminal domain (Nab2-N). Here, we present a structural and functional analysis of the Gfd1/Nab2-N interaction. Crystallography, supported by solution NMR, shows that Gfd1 residues 126-150 form an alpha-helix when bound to Nab2-N. Engineered Nab2-N and Gfd1 mutants that inhibit this interaction in vitro were used to probe its function in vivo using the genetic interaction between GFD1 and NAB2. Although GFD1 is not essential for viability, its deletion severely impairs growth of rat8-2 (dbp5) cells. Moreover, although Gfd1 overexpression suppresses rat8-2 (dbp5), Gfd1 mutants that do not bind Nab2 only partially suppress rat8-2 (dbp5). Furthermore, rat8-2 (dbp5) cells that express nab2-Y34A, in which binding to Gfd1 is impaired, show a synthetic growth phenotype and nuclear accumulation of poly(A) RNA. These data support the importance of the Gfd1/Nab2 interaction for Dbp5 activity and provide further molecular details of the interactions that facilitate Dbp5-mediated mRNP remodeling in the terminal step of mRNA export.
- MRC Laboratory of Molecular Biology, Hills Road, Cambridge CB2 0QH, United Kingdom.
Organizational Affiliation: