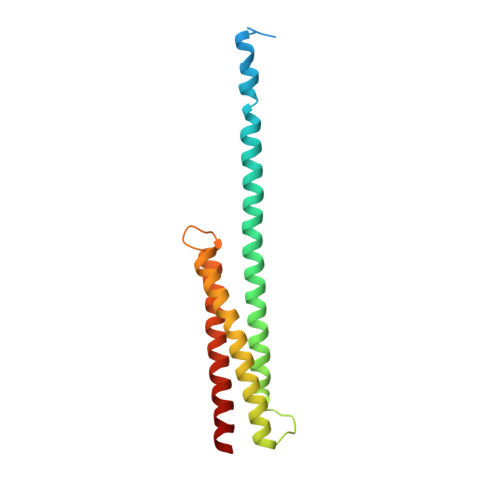
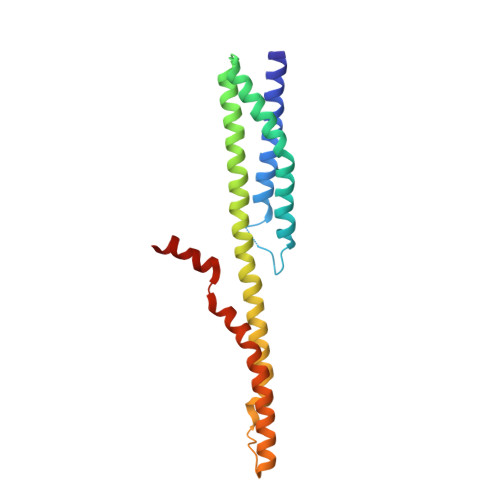
Crystal structure and functional interpretation of the erythrocyte spectrin tetramerization domain complex.
Ipsaro, J.J., Harper, S.L., Messick, T.E., Marmorstein, R., Mondragon, A., Speicher, D.W.(2010) Blood 115: 4843-4852
- PubMed: 20197550
- DOI: https://doi.org/10.1182/blood-2010-01-261396
- Primary Citation of Related Structures:
3LBX - PubMed Abstract:
As the principal component of the membrane skeleton, spectrin confers integrity and flexibility to red cell membranes. Although this network involves many interactions, the most common hemolytic anemia mutations that disrupt erythrocyte morphology affect the spectrin tetramerization domains. Although much is known clinically about the resulting conditions (hereditary elliptocytosis and pyropoikilocytosis), the detailed structural basis for spectrin tetramerization and its disruption by hereditary anemia mutations remains elusive. Thus, to provide further insights into spectrin assembly and tetramer site mutations, a crystal structure of the spectrin tetramerization domain complex has been determined. Architecturally, this complex shows striking resemblance to multirepeat spectrin fragments, with the interacting tetramer site region forming a central, composite repeat. This structure identifies conformational changes in alpha-spectrin that occur upon binding to beta-spectrin, and it reports the first structure of the beta-spectrin tetramerization domain. Analysis of the interaction surfaces indicates an extensive interface dominated by hydrophobic contacts and supplemented by electrostatic complementarity. Analysis of evolutionarily conserved residues suggests additional surfaces that may form important interactions. Finally, mapping of hereditary anemia-related mutations onto the structure demonstrate that most, but not all, local hereditary anemia mutations map to the interacting domains. The potential molecular effects of these mutations are described.
- Department of Biochemistry, Molecular Biology, and Cell Biology, Northwestern University, Evanston, IL 60208, USA.
Organizational Affiliation: