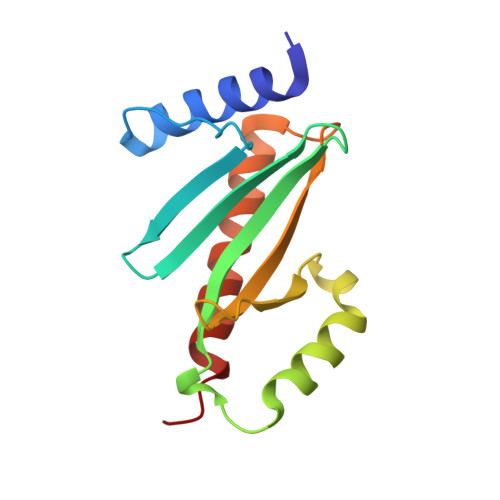
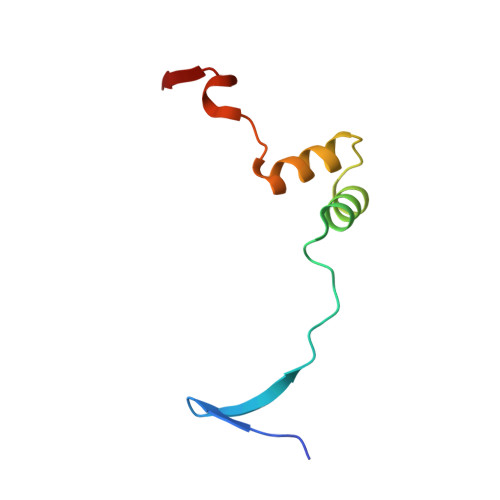
Analysis of the Crystal Structure of the ExsC.ExsE Complex Reveals Distinctive Binding Interactions of the Pseudomonas aeruginosa Type III Secretion Chaperone ExsC with ExsE and ExsD.
Vogelaar, N.J., Jing, X., Robinson, H.H., Schubot, F.D.(2010) Biochemistry 49: 5870-5879
- PubMed: 20536183
- DOI: https://doi.org/10.1021/bi100432e
- Primary Citation of Related Structures:
3KXY - PubMed Abstract:
Pseudomonas aeruginosa, like many Gram-negative bacterial pathogens, requires its type III secretion system (T3SS) to facilitate acute infections. In P. aeruginosa, the expression of all T3SS-related genes is regulated by the transcriptional activator ExsA. A signaling cascade involving ExsA and three additional proteins, ExsC, ExsD, and ExsE, directly ties the upregulation of ExsA-mediated transcription to the activation of the type III secretion apparatus. In order to characterize the events underlying the signaling process, the crystal structure of the T3SS chaperone ExsC in complex with its cognate effector ExsE has been determined. The structure reveals critical contacts that mediate the interactions between these two proteins. Particularly striking is the presence of two Arg-X-Val-X-Arg motifs in ExsE that form identical interactions along opposite sides of an ExsC dimer. The structure also provides insights into the interactions of ExsC with the antiactivator protein ExsD. It was shown that the amino-terminal 46 residues of ExsD are sufficient for ExsC binding. On the basis of these findings, a new model for the ExsC.ExsD complex is proposed to explain its distinctive 2:2 stoichiometry and why ExsC displays a weaker affinity for ExsD than for ExsE.
- Department of Biological Sciences, Life Science I, Virginia Polytechnic Institute and State University, Washington Street, Blacksburg, Virginia 24060, USA.
Organizational Affiliation: