The crystal structure of the mutant K300M of polyamine oxidase from ZEA MAYS unveils the role of LYS300 in catalysis
Fiorillo, A., Ilari, A., Tavladoraki, P.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Polyamine oxidase | 478 | Zea mays | Mutation(s): 1 Gene Names: PAO EC: 1.5.3 (PDB Primary Data), 1.5.3.14 (UniProt), 1.5.3.15 (UniProt) | 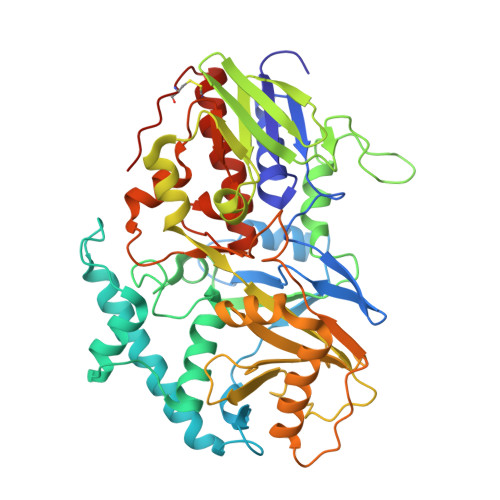 | |
UniProt | |||||
Find proteins for O64411 (Zea mays) Explore O64411 Go to UniProtKB: O64411 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O64411 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | |||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FAD Query on FAD | D [auth A], G [auth B] | FLAVIN-ADENINE DINUCLEOTIDE C27 H33 N9 O15 P2 VWWQXMAJTJZDQX-UYBVJOGSSA-N |  | ||
| NAG Query on NAG | E [auth A] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| SPM Query on SPM | F [auth A], H [auth B] | SPERMINE C10 H26 N4 PFNFFQXMRSDOHW-UHFFFAOYSA-N |  | ||
| SO4 Query on SO4 | J [auth B] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| CL Query on CL | I [auth B] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 138.957 | α = 90 |
| b = 138.957 | β = 90 |
| c = 189.8 | γ = 120 |
| Software Name | Purpose |
|---|---|
| MOLREP | phasing |
| REFMAC | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |