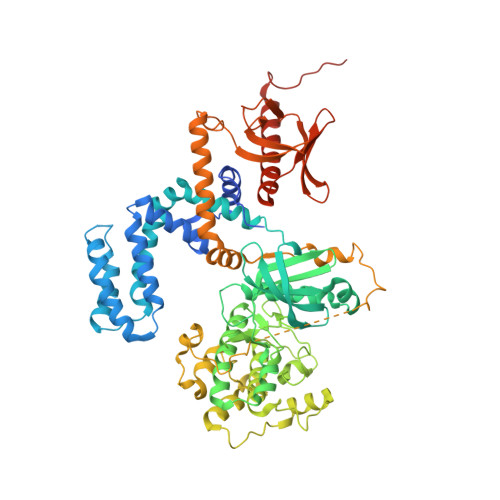
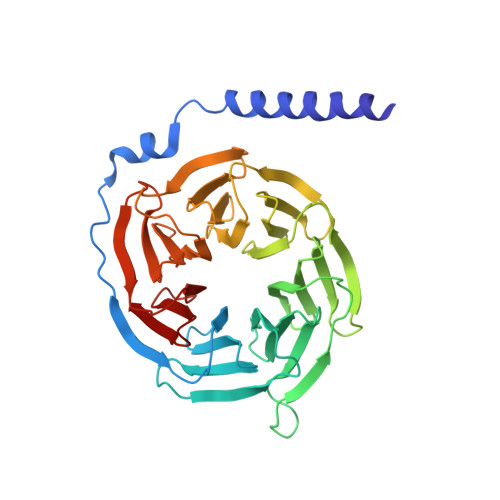
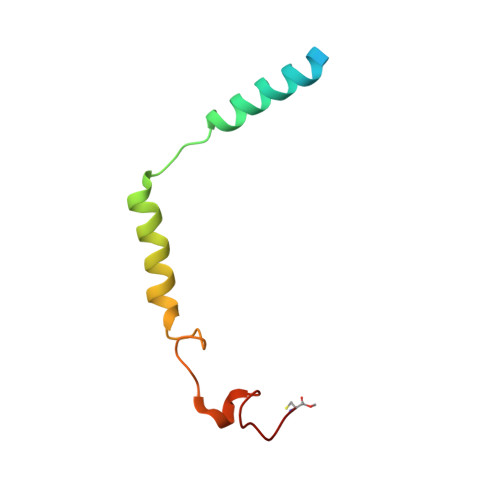
Structure of human g protein-coupled receptor kinase 2 in complex with the kinase inhibitor balanol.
Tesmer, J.J., Tesmer, V.M., Lodowski, D.T., Steinhagen, H., Huber, J.(2010) J Med Chem 53: 1867-1870
- PubMed: 20128603
- DOI: https://doi.org/10.1021/jm9017515
- Primary Citation of Related Structures:
3CIK, 3KRW, 3KRX - PubMed Abstract:
G protein-coupled receptor kinase 2 (GRK2) is a pharmaceutical target for the treatment of cardiovascular diseases such as congestive heart failure, myocardial infarction, and hypertension. To better understand how nanomolar inhibition and selectivity for GRK2 might be achieved, we have determined crystal structures of human GRK2 in complex with Gbetagamma in the presence and absence of the AGC kinase inhibitor balanol. The selectivity of balanol among human GRKs is assessed.
- Life Sciences Institute, University of Michigan, Ann Arbor, Michigan 48109-2216, USA. johntesmer@umich.edu
Organizational Affiliation: