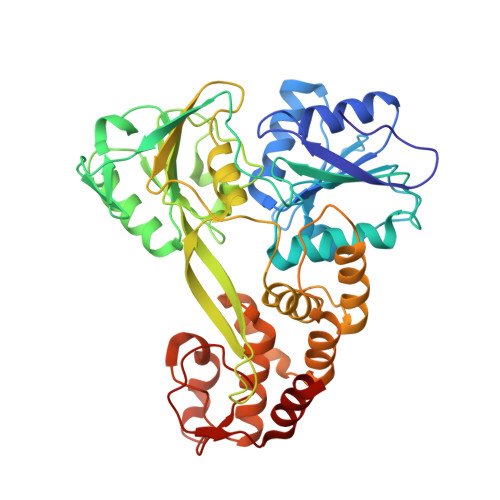
Inaugural Article: Three conformational snapshots of the hepatitis C virus NS3 helicase reveal a ratchet translocation mechanism.
Gu, M., Rice, C.M.(2010) Proc Natl Acad Sci U S A 107: 521-528
- PubMed: 20080715
- DOI: https://doi.org/10.1073/pnas.0913380107
- Primary Citation of Related Structures:
3KQH, 3KQK, 3KQL, 3KQN, 3KQU - PubMed Abstract:
A virally encoded superfamily-2 (SF2) helicase (NS3h) is essential for the replication of hepatitis C virus, a leading cause of liver disease worldwide. Efforts to elucidate the function of NS3h and to develop inhibitors against it, however, have been hampered by limited understanding of its molecular mechanism. Here we show x-ray crystal structures for a set of NS3h complexes, including ground-state and transition-state ternary complexes captured with ATP mimics (ADP.BeF(3) and ). These structures provide, for the first time, three conformational snapshots demonstrating the molecular basis of action for a SF2 helicase. Upon nucleotide binding, overall domain rotation along with structural transitions in motif V and the bound DNA leads to the release of one base from the substrate base-stacking row and the loss of several interactions between NS3h and the 3' DNA segment. As nucleotide hydrolysis proceeds into the transition state, stretching of a "spring" helix and another overall conformational change couples rearrangement of the (d)NTPase active site to additional hydrogen-bonding between NS3h and DNA. Together with biochemistry, these results demonstrate a "ratchet" mechanism involved in the unidirectional translocation and define the step size of NS3h as one base per nucleotide hydrolysis cycle. These findings suggest feasible strategies for developing specific inhibitors to block the action of this attractive, yet largely unexplored drug target.
- Laboratory of Virology and Infectious Disease, Center for the Study of Hepatitis C, The Rockefeller University, New York, NY 10065, USA. mgu@rockefeller.edu
Organizational Affiliation: