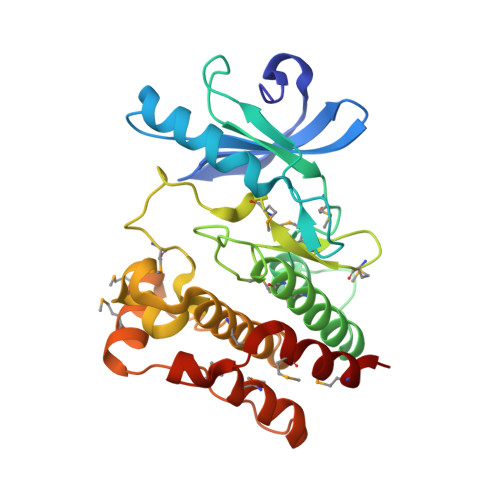
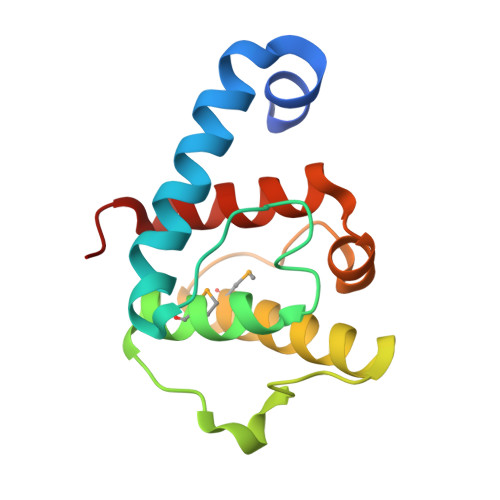
The pseudoactive site of ILK is essential for its binding to alpha-Parvin and localization to focal adhesions.
Fukuda, K., Gupta, S., Chen, K., Wu, C., Qin, J.(2009) Mol Cell 36: 819-830
- PubMed: 20005845
- DOI: https://doi.org/10.1016/j.molcel.2009.11.028
- Primary Citation of Related Structures:
3KMU, 3KMW - PubMed Abstract:
Integrin-linked kinase (ILK) plays a pivotal role in connecting transmembrane receptor integrin to the actin cytoskeleton and thereby regulating diverse cell-adhesion-dependent processes. The kinase domain (KD) of ILK is indispensable for its function, but the underlying molecular basis remains enigmatic. Here we present the crystal structure of the ILK KD bound to its cytoskeletal regulator, the C-terminal calponin homology domain of alpha-parvin. While maintaining a canonical kinase fold, the ILK KD displays a striking pseudoactive site conformation. We show that rather than performing the kinase function, this conformation specifically recognizes alpha-parvin for promoting effective assembly of ILK into focal adhesions. The alpha-parvin-bound ILK KD can simultaneously engage integrin beta cytoplasmic tails. These results thus define ILK as a distinct pseudokinase that mechanically couples integrin and alpha-parvin for mediating cell adhesion. They also highlight functional diversity of the kinase fold and its "active" site in mediating many biological processes.
- Department of Molecular Cardiology, Lerner Research Institute, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Organizational Affiliation: