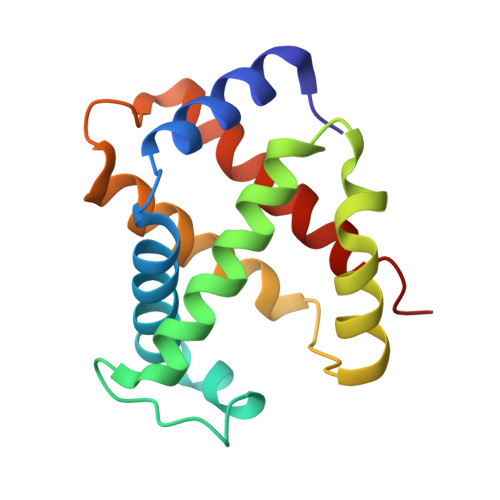
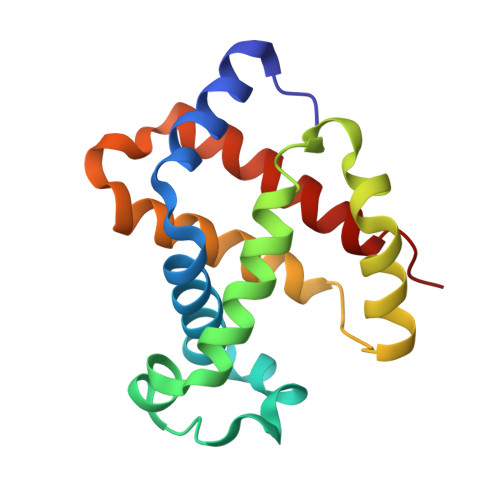
Direct Determination of Protonation States of Histidine Residues in a 2 A Neutron Structure of Deoxy-Human Normal Adult Hemoglobin and Implications for the Bohr Effect.
Kovalevsky, A.Y., Chatake, T., Shibayama, N., Park, S.Y., Ishikawa, T., Mustyakimov, M., Fisher, Z., Langan, P., Morimoto, Y.(2010) J Mol Biology 398: 276-291
- PubMed: 20230836
- DOI: https://doi.org/10.1016/j.jmb.2010.03.016
- Primary Citation of Related Structures:
3KMF - PubMed Abstract:
We have investigated the protonation states of histidine residues (potential Bohr groups) in the deoxy form (T state) of human hemoglobin by direct determination of hydrogen (deuterium) positions with the neutron protein crystallography technique. The reversible binding of protons is key to the allosteric regulation of human hemoglobin. The protonation states of 35 of the 38 His residues were directly determined from neutron scattering omit maps, with 3 of the remaining residues being disordered. Protonation states of 5 equivalent His residues--alpha His20, alpha His50, alpha His89, beta His143, and beta His146--differ between the symmetry-related globin subunits. The distal His residues, alpha His58 and beta His63, are protonated in the alpha 1 beta 1 heterodimer and are neutral in alpha 2 beta 2. Buried residue alpha His103 is found to be protonated in both subunits. These distal and buried residues have the potential to act as Bohr groups. The observed protonation states of His residues are compared to changes in their pK(a) values during the transition from the T to the R state and the results provide some new insights into our understanding of the molecular mechanism of the Bohr effect.
- Bioscience Division, Los Alamos National Laboratory, Los Alamos, NM 87545, USA. ayk@lanl.gov
Organizational Affiliation: