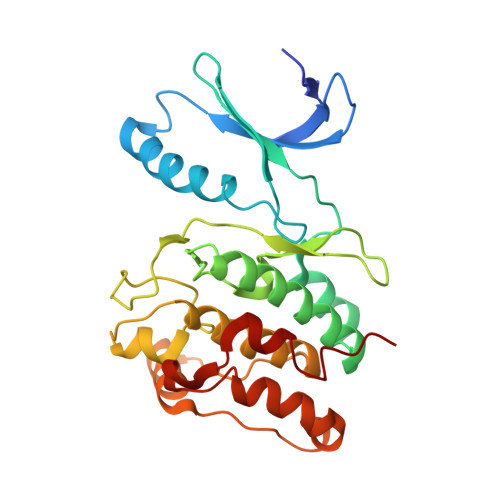
Intersubunit capture of regulatory segments is a component of cooperative CaMKII activation.
Chao, L.H., Pellicena, P., Deindl, S., Barclay, L.A., Schulman, H., Kuriyan, J.(2010) Nat Struct Mol Biol 17: 264-272
- PubMed: 20139983
- DOI: https://doi.org/10.1038/nsmb.1751
- Primary Citation of Related Structures:
3KK8, 3KK9, 3KL8 - PubMed Abstract:
The dodecameric holoenzyme of calcium-calmodulin-dependent protein kinase II (CaMKII) responds to high-frequency Ca(2+) pulses to become Ca(2+) independent. A simple coincidence-detector model for Ca(2+)-frequency dependency assumes noncooperative activation of kinase domains. We show that activation of CaMKII by Ca(2+)-calmodulin is cooperative, with a Hill coefficient of approximately 3.0, implying sequential kinase-domain activation beyond dimeric units. We present data for a model in which cooperative activation includes the intersubunit 'capture' of regulatory segments. Such a capture interaction is seen in a crystal structure that shows extensive contacts between the regulatory segment of one kinase and the catalytic domain of another. These interactions are mimicked by a natural inhibitor of CaMKII. Our results show that a simple coincidence-detection model cannot be operative and point to the importance of kinetic dissection of the frequency-response mechanism in future experiments.
- Department of Molecular and Cell Biology, University of California, Berkeley, California, USA.
Organizational Affiliation: