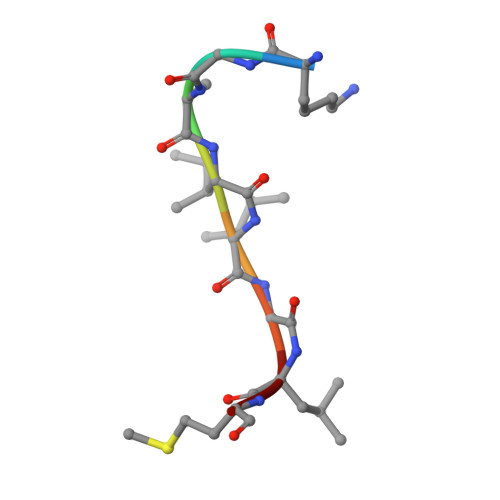
Crystal structure of the complex formed between Phospholipase A2 with beta-amyloid fragment, Lys-Gly-Ala-Ile-Ile-Gly-Leu-Met at 1.8 A resolution
Pandey, N., Mirza, Z., Vikram, G., Singh, N., Bhushan, A., Kaur, P., Srinivasan, A., Sharma, S., Singh, T.P.To be published.