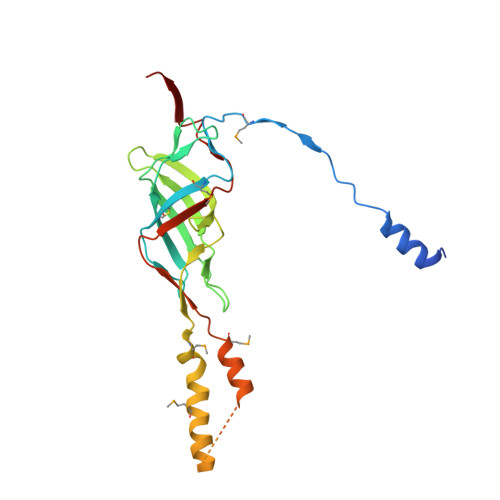
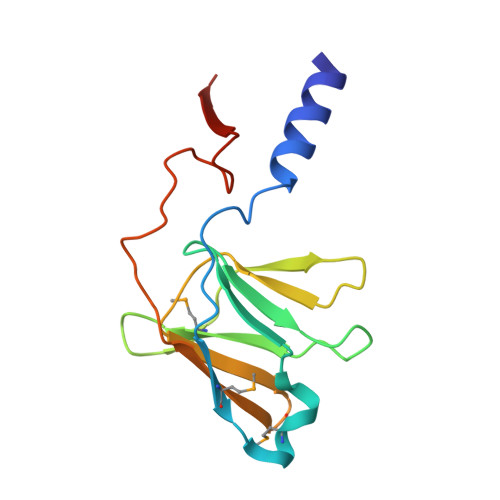
Structure of the outer membrane complex of a type IV secretion system
Chandran, V., Fronzes, R., Duquerroy, S., Cronin, N., Navaza, J., Waksman, G.(2009) Nature 462: 1011-1015
- PubMed: 19946264
- DOI: https://doi.org/10.1038/nature08588
- Primary Citation of Related Structures:
3JQO - PubMed Abstract:
Type IV secretion systems are secretion nanomachines spanning the two membranes of Gram-negative bacteria. Three proteins, VirB7, VirB9 and VirB10, assemble into a 1.05 megadalton (MDa) core spanning the inner and outer membranes. This core consists of 14 copies of each of the proteins and forms two layers, the I and O layers, inserting in the inner and outer membrane, respectively. Here we present the crystal structure of a approximately 0.6 MDa outer-membrane complex containing the entire O layer. This structure is the largest determined for an outer-membrane channel and is unprecedented in being composed of three proteins. Unexpectedly, this structure identifies VirB10 as the outer-membrane channel with a unique hydrophobic double-helical transmembrane region. This structure establishes VirB10 as the only known protein crossing both membranes of Gram-negative bacteria. Comparison of the cryo-electron microscopy (cryo-EM) and crystallographic structures points to conformational changes regulating channel opening and closing.
- Institute of Structural and Molecular Biology, University College London and Birkbeck College, Malet Street, London WC1E 7HX, UK.
Organizational Affiliation: