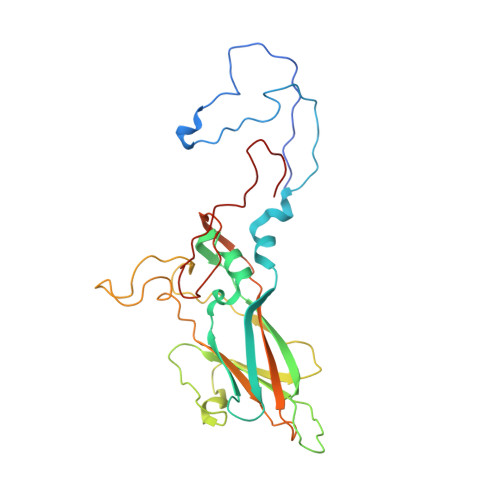
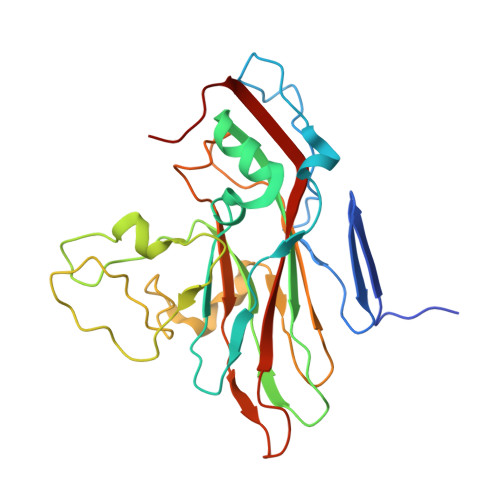
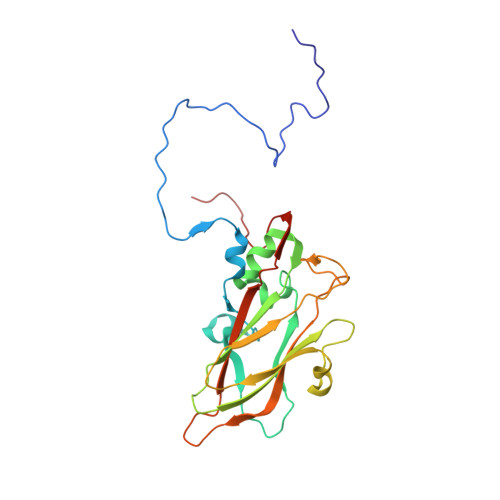
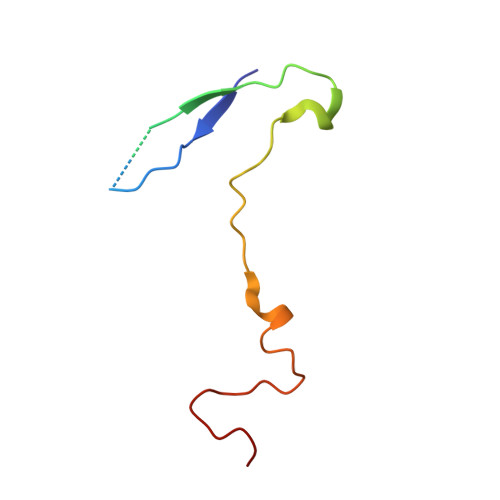
The novel asymmetric entry intermediate of a picornavirus captured with nanodiscs.
Lee, H., Shingler, K.L., Organtini, L.J., Ashley, R.E., Makhov, A.M., Conway, J.F., Hafenstein, S.(2016) Sci Adv 2: e1501929-e1501929
- PubMed: 27574701
- DOI: https://doi.org/10.1126/sciadv.1501929
- Primary Citation of Related Structures:
3JD7 - PubMed Abstract:
Many nonenveloped viruses engage host receptors that initiate capsid conformational changes necessary for genome release. Structural studies on the mechanisms of picornavirus entry have relied on in vitro approaches of virus incubated at high temperatures or with excess receptor molecules to trigger the entry intermediate or A-particle. We have induced the coxsackievirus B3 entry intermediate by triggering the virus with full-length receptors embedded in lipid bilayer nanodiscs. These asymmetrically formed A-particles were reconstructed using cryo-electron microscopy and a direct electron detector. These first high-resolution structures of a picornavirus entry intermediate captured at a membrane with and without imposing icosahedral symmetry (3.9 and 7.8 Å, respectively) revealed a novel A-particle that is markedly different from the classical A-particles. The asymmetric receptor binding triggers minimal global capsid expansion but marked local conformational changes at the site of receptor interaction. In addition, viral proteins extrude from the capsid only at the site of extensive protein remodeling adjacent to the nanodisc. Thus, the binding of the receptor triggers formation of a unique site in preparation for genome release.
- The Pennsylvania State University College of Medicine, Hershey, PA 17033, USA.
Organizational Affiliation: