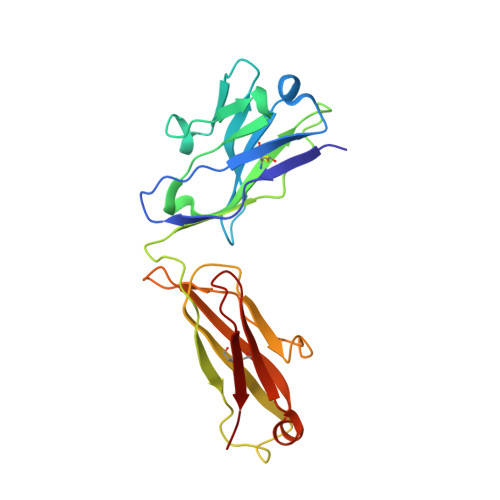
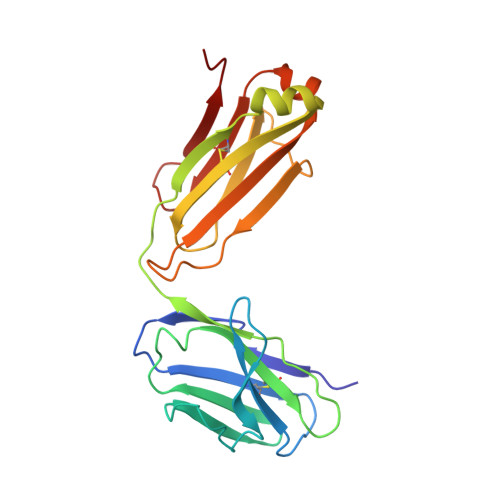
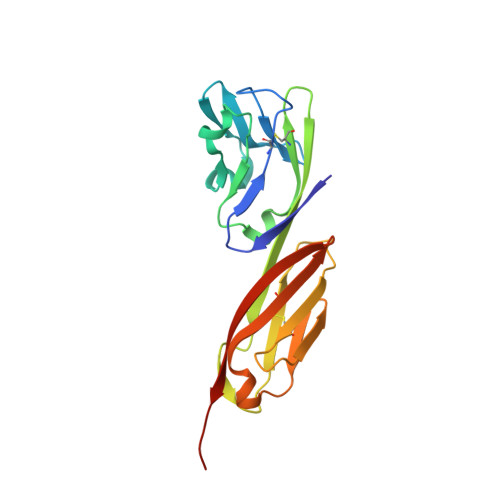
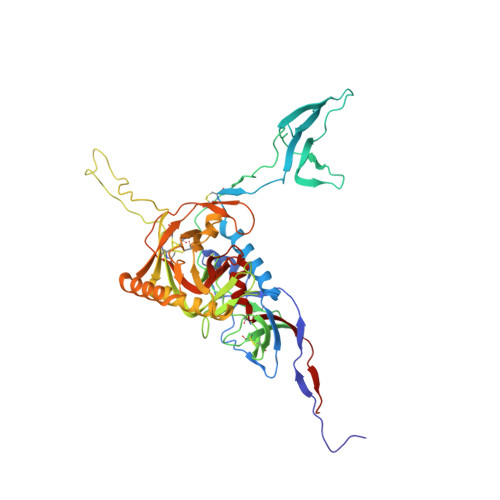
Computational Refinement and Validation Protocol for Proteins with Large Variable Regions Applied to Model HIV Env Spike in CD4 and 17b Bound State.
Rasheed, M., Bettadapura, R., Bajaj, C.(2015) Structure 23: 1138-1149
- PubMed: 26039348
- DOI: https://doi.org/10.1016/j.str.2015.03.026
- Primary Citation of Related Structures:
3J70 - PubMed Abstract:
Envelope glycoprotein gp120 of HIV-1 possesses several variable regions; their precise structure has been difficult to establish. We report a new model of gp120, in complex with antibodies CD4 and 17b, complete with its variable regions. The model was produced by a computational protocol that uses cryo-electron microscopy (EM) maps, atomic-resolution structures of the core, and information on binding interactions. Our model has excellent fit with EMD: 5020, is stereochemically and energetically favorable, and has the expected binding interfaces. Comparison of the ternary arrangement of the loops in this model with those bound to PGT122 and PGV04 suggested a possible motion of the V1V2 away from the CCR5 binding site and toward CD4. Our study also revealed that the CD4-bound state of the V1V2 loop is not optimal for gp120 bound with several neutralizing antibodies.
- Computer Science Department, The University of Texas at Austin, 1 University Station, Austin, TX 78712, USA.
Organizational Affiliation: