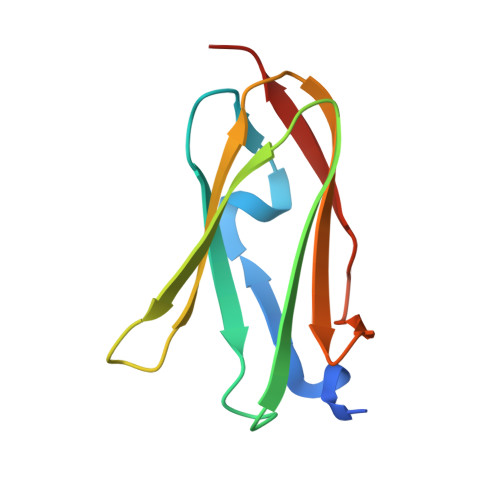
Biochemical basis of the interaction between cystic fibrosis transmembrane conductance regulator and immunoglobulin-like repeats of filamin.
Smith, L., Page, R.C., Xu, Z., Kohli, E., Litman, P., Nix, J.C., Ithychanda, S.S., Liu, J., Qin, J., Misra, S., Liedtke, C.M.(2010) J Biological Chem 285: 17166-17176
- PubMed: 20351101
- DOI: https://doi.org/10.1074/jbc.M109.080911
- Primary Citation of Related Structures:
3ISW - PubMed Abstract:
Mutations in the chloride channel cystic fibrosis transmembrane regulator (CFTR) cause cystic fibrosis, a genetic disorder characterized by defects in CFTR biosynthesis, localization to the cell surface, or activation by regulatory factors. It was discovered recently that surface localization of CFTR is stabilized by an interaction between the CFTR N terminus and the multidomain cytoskeletal protein filamin. The details of the CFTR-filamin interaction, however, are unclear. Using x-ray crystallography, we show how the CFTR N terminus binds to immunoglobulin-like repeat 21 of filamin A (FlnA-Ig21). CFTR binds to beta-strands C and D of FlnA-Ig21 using backbone-backbone hydrogen bonds, a linchpin serine residue, and hydrophobic side-chain packing. We use NMR to determine that the CFTR N terminus also binds to several other immunoglobulin-like repeats from filamin A in vitro. Our structural data explain why the cystic fibrosis-causing S13F mutation disrupts CFTR-filamin interaction. We show that FlnA-Ig repeats transfected into cultured Calu-3 cells disrupt CFTR-filamin interaction and reduce surface levels of CFTR. Our findings suggest that filamin A stabilizes surface CFTR by anchoring it to the actin cytoskeleton through interactions with multiple filamin Ig repeats. Such an interaction mode may allow filamins to cluster multiple CFTR molecules and to promote colocalization of CFTR and other filamin-binding proteins in the apical plasma membrane of epithelial cells.
- Department of Pediatrics at Rainbow Babies and Children's Hospital and of Physiology and Biophysics, Case Western Reserve University, Willard Alan Bernbaum Center for Cystic Fibrosis Research, Cleveland, Ohio 44106, USA.
Organizational Affiliation: