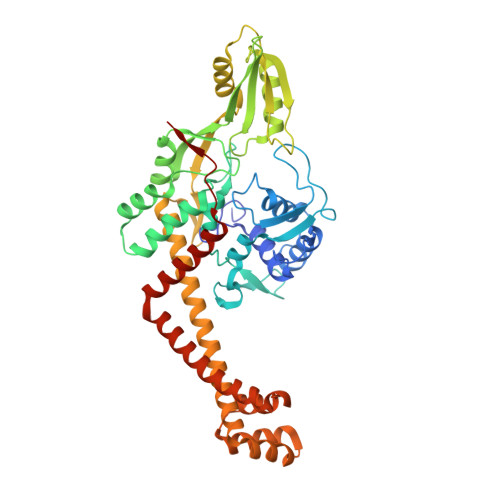
Crystal structure of the DNA gyrase GyrA N-terminal domain from Mycobacterium tuberculosis.
Tretter, E.M., Schoeffler, A.J., Weisfield, S.R., Berger, J.M.(2010) Proteins 78: 492-495
- PubMed: 19787774
- DOI: https://doi.org/10.1002/prot.22600
- Primary Citation of Related Structures:
3ILW - Division of Biochemistry and Molecular Biology, Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, California 94720-3220, USA.
Organizational Affiliation: