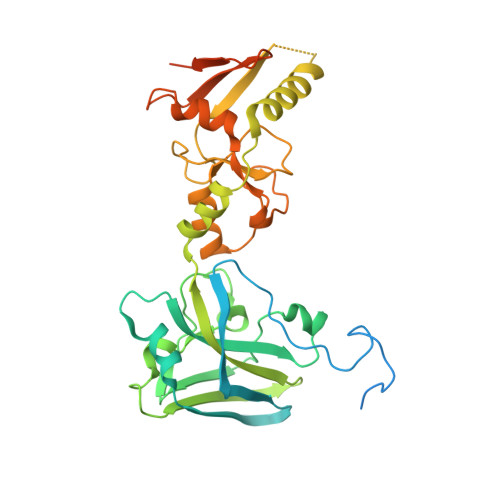
Structure of the Siz/PIAS SUMO E3 ligase Siz1 and determinants required for SUMO modification of PCNA.
Yunus, A.A., Lima, C.D.(2009) Mol Cell 35: 669-682
- PubMed: 19748360
- DOI: https://doi.org/10.1016/j.molcel.2009.07.013
- Primary Citation of Related Structures:
3I2D - PubMed Abstract:
Siz1 is a founding member of the Siz/PIAS RING family of SUMO E3 ligases. The X-ray structure of an active Siz1 ligase revealed an elongated tripartite architecture comprised of an N-terminal PINIT domain, a central zinc-containing RING-like SP-RING domain, and a C-terminal domain we term the SP-CTD. Structure-based mutational analysis and biochemical studies show that the SP-RING and SP-CTD are required for activation of the E2 approximately SUMO thioester, while the PINIT domain is essential for redirecting SUMO conjugation to the proliferating cell nuclear antigen (PCNA) at lysine 164, a nonconsensus lysine residue that is not modified by the SUMO E2 in the absence of Siz1. Mutational analysis of Siz1 and PCNA revealed surfaces on both proteins that are required for efficient SUMO modification of PCNA in vitro and in vivo.
- Sloan-Kettering Institute, New York, NY 10065, USA.
Organizational Affiliation: