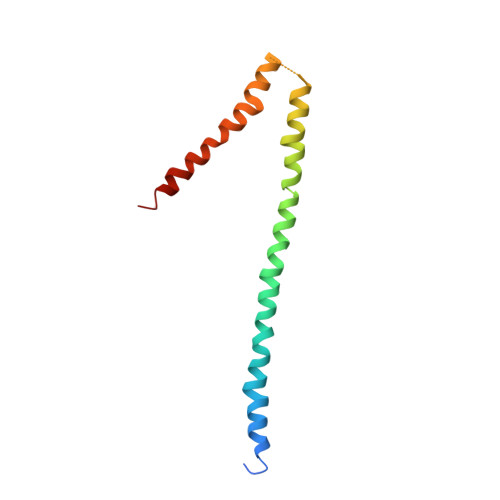Structural determination of functional units of the nucleotide binding domain (NBD94) of the reticulocyte binding protein Py235 of Plasmodium yoelii
Gruber, A., Manimekalai, M.S.S., Balakrishna, A.M., Hunke, C., Jeyakanthan, J., Preiser, P.R., Gruber, G.(2010) PLoS One 5: e9146-e9146
- PubMed: 20161776
- DOI: https://doi.org/10.1371/journal.pone.0009146
- Primary Citation of Related Structures:
3HGF - PubMed Abstract:
Invasion of the red blood cells (RBC) by the merozoite of malaria parasites involves a large number of receptor ligand interactions. The reticulocyte binding protein homologue family (RH) plays an important role in erythrocyte recognition as well as virulence. Recently, it has been shown that members of RH in addition to receptor binding may also have a role as ATP/ADP sensor. A 94 kDa region named Nucleotide-Binding Domain 94 (NBD94) of Plasmodium yoelii YM, representative of the putative nucleotide binding region of RH, has been demonstrated to bind ATP and ADP selectively. Binding of ATP or ADP induced nucleotide-dependent structural changes in the C-terminal hinge-region of NBD94, and directly impacted on the RBC binding ability of RH. In order to find the smallest structural unit, able to bind nucleotides, and its coupling module, the hinge region, three truncated domains of NBD94 have been generated, termed NBD94(444-547), NBD94(566-663) and NBD94(674-793), respectively. Using fluorescence correlation spectroscopy NBD94(444-547) has been identified to form the smallest nucleotide binding segment, sensitive for ATP and ADP, which became inhibited by 4-Chloro-7-nitrobenzofurazan. The shape of NBD94(444-547) in solution was calculated from small-angle X-ray scattering data, revealing an elongated molecule, comprised of two globular domains, connected by a spiral segment of about 73.1 A in length. The high quality of the constructs, forming the hinge-region, NBD94(566-663) and NBD94(674-793) enabled to determine the first crystallographic and solution structure, respectively. The crystal structure of NBD94(566-663) consists of two helices with 97.8 A and 48.6 A in length, linked by a loop. By comparison, the low resolution structure of NBD94(674-793) in solution represents a chair-like shape with three architectural segments. These structures give the first insight into how nucleotide binding impacts on the overall structure of RH and demonstrates the potential use of this region as a novel drug target.
- School of Biological Sciences, Nanyang Technological University, Singapore. ggrueber@ntu.edu.sg
Organizational Affiliation: