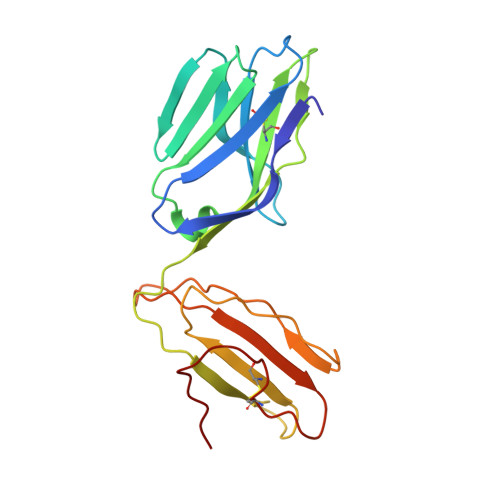
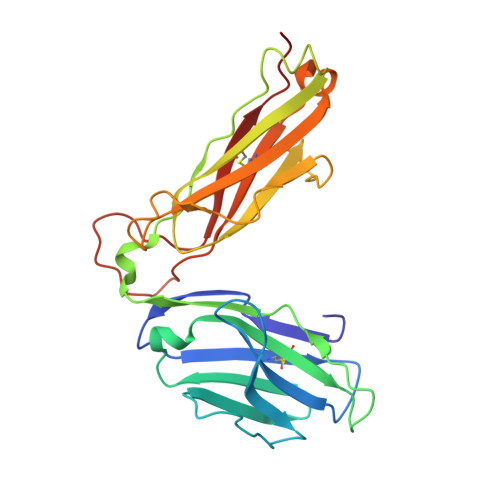
Germ line-governed recognition of a cancer epitope by an immunodominant human T-cell receptor.
Cole, D.K., Yuan, F., Rizkallah, P.J., Miles, J.J., Gostick, E., Price, D.A., Gao, G.F., Jakobsen, B.K., Sewell, A.K.(2009) J Biological Chem 284: 27281-27289
- PubMed: 19605354
- DOI: https://doi.org/10.1074/jbc.M109.022509
- Primary Citation of Related Structures:
3HG1 - PubMed Abstract:
CD8(+) T-cells specific for MART-1-(26-35), a dominant melanoma epitope restricted by human leukocyte antigen (HLA)-A*0201, are exceptionally common in the naive T-cell repertoire. Remarkably, the TRAV12-2 gene is used to encode the T-cell receptor alpha (TCRalpha) chain in >87% of these T-cells. Here, the molecular basis for this genetic bias is revealed from the structural and thermodynamic properties of an archetypal TRAV12-2-encoded TCR complexed to the clinically relevant heteroclitic peptide, ELAGIGILTV, bound to HLA-A*0201 (A2-ELA). Unusually, the TRAV12-2 germ line-encoded regions of the TCR dominate the major atomic contacts with the peptide at the TCR/A2-ELA interface. This "innate" pattern of antigen recognition probably explains the unique characteristics and extraordinary frequencies of CD8(+) T-cell responses to this epitope.
- Department of Infection, Immunity, and Biochemistry, Henry Wellcome Building, Cardiff University School of Medicine, Heath Park, Cardiff CF14 4XN, United Kingdom.
Organizational Affiliation: