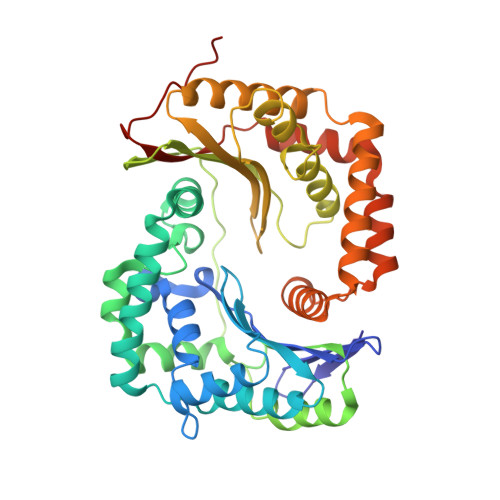
Crystal and solution structures of a prokaryotic M16B peptidase: an open and shut case.
Aleshin, A.E., Gramatikova, S., Hura, G.L., Bobkov, A., Strongin, A.Y., Stec, B., Tainer, J.A., Liddington, R.C., Smith, J.W.(2009) Structure 17: 1465-1475
- PubMed: 19913481
- DOI: https://doi.org/10.1016/j.str.2009.09.009
- Primary Citation of Related Structures:
3HDI - PubMed Abstract:
The M16 family of zinc peptidases comprises a pair of homologous domains that form two halves of a "clam-shell" surrounding the active site. The M16A and M16C subfamilies form one class ("peptidasomes"): they degrade 30-70 residue peptides, and adopt both open and closed conformations. The eukaryotic M16B subfamily forms a second class ("processing proteases"): they adopt a single partly-open conformation that enables them to cleave signal sequences from larger proteins. Here, we report the solution and crystal structures of a prokaryotic M16B peptidase, and demonstrate that it has features of both classes: thus, it forms stable "open" homodimers in solution that resemble the processing proteases; but the clam-shell closes upon binding substrate, a feature of the M16A/C peptidasomes. Moreover, clam-shell closure is required for proteolytic activity. We predict that other prokaryotic M16B family members will form dimeric peptidasomes, and propose a model for the evolution of the M16 family.
- Burnham Institute for Medical Research, La Jolla, CA 92037, USA.
Organizational Affiliation: