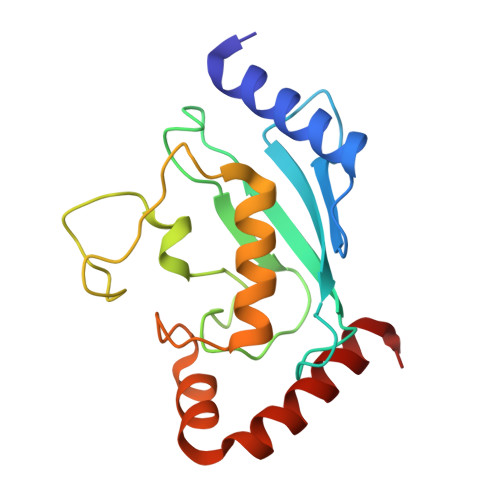
Allosteric activation of E2-RING finger-mediated ubiquitylation by a structurally defined specific E2-binding region of gp78.
Das, R., Mariano, J., Tsai, Y.C., Kalathur, R.C., Kostova, Z., Li, J., Tarasov, S.G., McFeeters, R.L., Altieri, A.S., Ji, X., Byrd, R.A., Weissman, A.M.(2009) Mol Cell 34: 674-685
- PubMed: 19560420
- DOI: https://doi.org/10.1016/j.molcel.2009.05.010
- Primary Citation of Related Structures:
3H8K - PubMed Abstract:
The activity of RING finger ubiquitin ligases (E3) is dependent on their ability to facilitate transfer of ubiquitin from ubiquitin-conjugating enzymes (E2) to substrates. The G2BR domain within the E3 gp78 binds selectively and with high affinity to the E2 Ube2g2. Through structural and functional analyses, we determine that this occurs on a region of Ube2g2 distinct from binding sites for ubiquitin-activating enzyme (E1) and RING fingers. Binding to the G2BR results in conformational changes in Ube2g2 that affect ubiquitin loading. The Ube2g2:G2BR interaction also causes an approximately 50-fold increase in affinity between the E2 and RING finger. This results in markedly increased ubiquitylation by Ube2g2 and the gp78 RING finger. The significance of this G2BR effect is underscored by enhanced ubiquitylation observed when Ube2g2 is paired with other RING finger E3s. These findings uncover a mechanism whereby allosteric effects on an E2 enhance E2-RING finger interactions and, consequently, ubiquitylation.
- Structural Biophysics Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, MD 21702-1201, USA.
Organizational Affiliation: