From the Cover: A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates.
Chan, J.C., Piper, D.E., Cao, Q., Liu, D., King, C., Wang, W., Tang, J., Liu, Q., Higbee, J., Xia, Z., Di, Y., Shetterly, S., Arimura, Z., Salomonis, H., Romanow, W.G., Thibault, S.T., Zhang, R., Cao, P., Yang, X.P., Yu, T., Lu, M., Retter, M.W., Kwon, G., Henne, K., Pan, O., Tsai, M.M., Fuchslocher, B., Yang, E., Zhou, L., Lee, K.J., Daris, M., Sheng, J., Wang, Y., Shen, W.D., Yeh, W.C., Emery, M., Walker, N.P., Shan, B., Schwarz, M., Jackson, S.M.(2009) Proc Natl Acad Sci U S A 106: 9820-9825
- PubMed: 19443683
- DOI: https://doi.org/10.1073/pnas.0903849106
- Primary Citation of Related Structures:
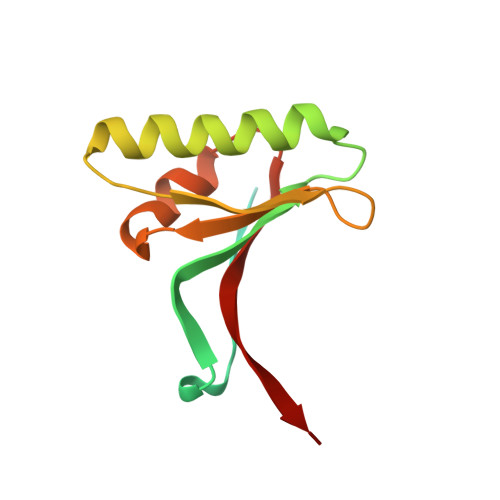
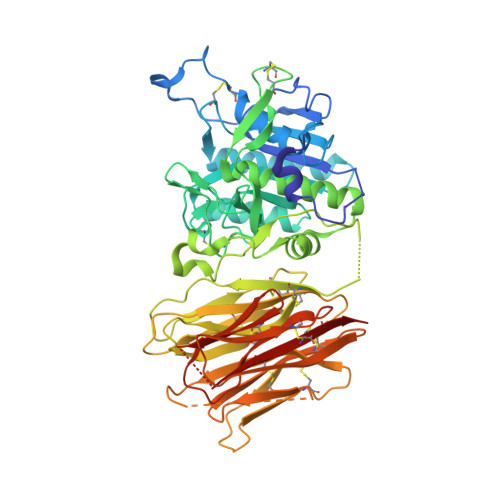
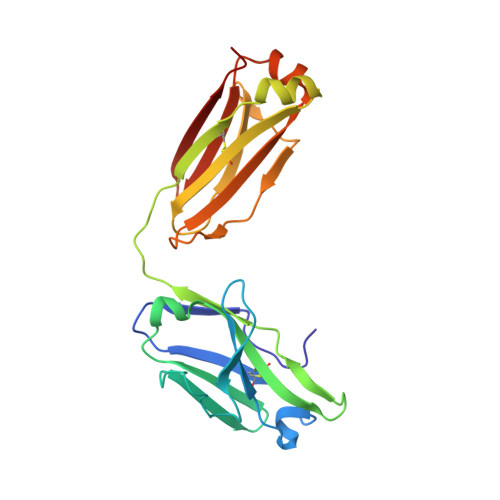
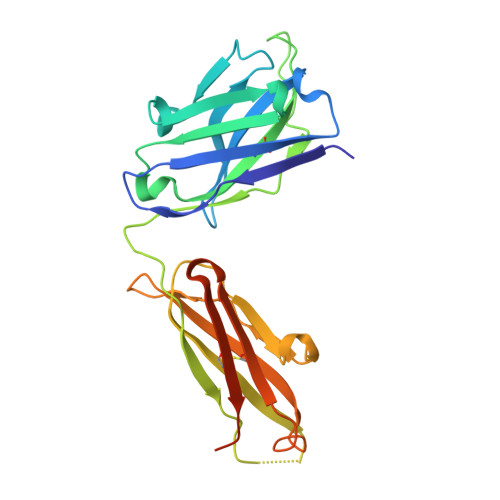
3H42 - PubMed Abstract:
Proprotein convertase subtilisin/kexin type 9 (PCSK9) regulates serum LDL cholesterol (LDL-C) by interacting with the LDL receptor (LDLR) and is an attractive therapeutic target for LDL-C lowering. We have generated a neutralizing anti-PCSK9 antibody, mAb1, that binds to an epitope on PCSK9 adjacent to the region required for LDLR interaction. In vitro, mAb1 inhibits PCSK9 binding to the LDLR and attenuates PCSK9-mediated reduction in LDLR protein levels, thereby increasing LDL uptake. A combination of mAb1 with a statin increases LDLR levels in HepG2 cells more than either treatment alone. In wild-type mice, mAb1 increases hepatic LDLR protein levels approximately 2-fold and lowers total serum cholesterol by up to 36%: this effect is not observed in LDLR(-/-) mice. In cynomolgus monkeys, a single injection of mAb1 reduces serum LDL-C by 80%, and a significant decrease is maintained for 10 days. We conclude that anti-PCSK9 antibodies may be effective therapeutics for treating hypercholesterolemia.
- Department of Metabolic Disorders, Amgen Inc., South San Francisco, CA 94080, USA.
Organizational Affiliation: