Crystal structure of mannose 6-phosphate isomerase fromSalmonella typhimurium bound to metal atoms and substrate: Implications for catalytic mechanism
Sagurthi, S.R., Giri, G., Savithri, H.S., Murthy, M.R.N.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Mannose-6-phosphate isomerase | 393 | Salmonella enterica subsp. enterica serovar Typhimurium | Mutation(s): 0 Gene Names: AAL20387, manA, pmi, STM1467 EC: 5.3.1.8 | 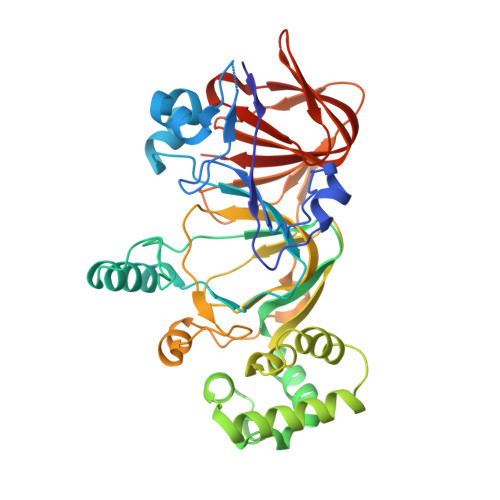 | |
UniProt | |||||
Find proteins for P25081 (Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720)) Explore P25081 Go to UniProtKB: P25081 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P25081 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| Y1 Query on Y1 | H [auth A] | YTTRIUM ION Y KAJPZYFHSCFBCI-UHFFFAOYSA-N |  | ||
| ZN Query on ZN | G [auth A] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | B [auth A], C [auth A], D [auth A], E [auth A], F [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 35.98 | α = 90 |
| b = 92.36 | β = 90 |
| c = 111.49 | γ = 90 |
| Software Name | Purpose |
|---|---|
| MAR345dtb | data collection |
| PHASER | phasing |
| REFMAC | refinement |
| MOSFLM | data reduction |
| SCALA | data scaling |