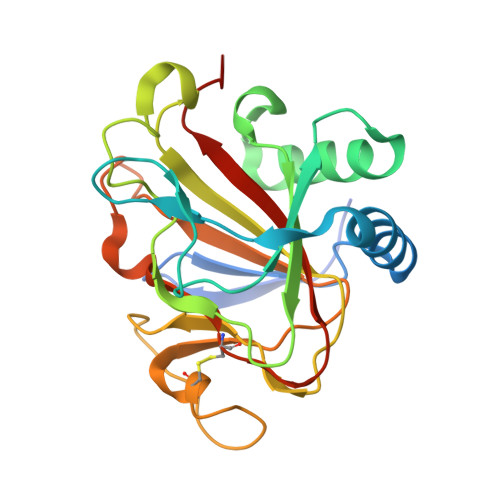
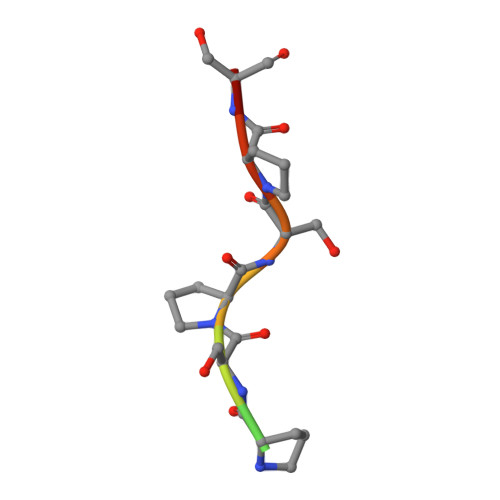
The Crystal Structure of an Algal Prolyl 4-Hydroxylase Complexed with a Proline-rich Peptide Reveals a Novel Buried Tripeptide Binding Motif
Koski, M.K., Hieta, R., Hirsila, M., Ronka, A., Myllyharju, J., Wierenga, R.K.(2009) J Biological Chem 284: 25290-25301
- PubMed: 19553701
- DOI: https://doi.org/10.1074/jbc.M109.014050
- Primary Citation of Related Structures:
3GZE - PubMed Abstract:
Plant and algal prolyl 4-hydroxylases (P4Hs) are key enzymes in the synthesis of cell wall components. These monomeric enzymes belong to the 2-oxoglutarate dependent superfamily of enzymes characterized by a conserved jelly-roll framework. This algal P4H has high sequence similarity to the catalytic domain of the vertebrate, tetrameric collagen P4Hs, whereas there are distinct sequence differences with the oxygen-sensing hypoxia-inducible factor P4H subfamily of enzymes. We present here a 1.98-A crystal structure of the algal Chlamydomonas reinhardtii P4H-1 complexed with Zn(2+) and a proline-rich (Ser-Pro)(5) substrate. This ternary complex captures the competent mode of binding of the peptide substrate, being bound in a left-handed (poly)l-proline type II conformation in a tunnel shaped by two loops. These two loops are mostly disordered in the absence of the substrate. The importance of these loops for the function is confirmed by extensive mutagenesis, followed up by enzyme kinetic characterizations. These loops cover the central Ser-Pro-Ser tripeptide of the substrate such that the hydroxylation occurs in a highly buried space. This novel mode of binding does not depend on stacking interactions of the proline side chains with aromatic residues. Major conformational changes of the two peptide binding loops are predicted to be a key feature of the catalytic cycle. These conformational changes are probably triggered by the conformational switch of Tyr(140), as induced by the hydroxylation of the proline residue. The importance of these findings for understanding the specific binding and hydroxylation of (X-Pro-Gly)(n) sequences by collagen P4Hs is also discussed.
- Biocenter Oulu and Department of Biochemistry, University of Oulu, FIN-90014 Oulu, Finland.
Organizational Affiliation: