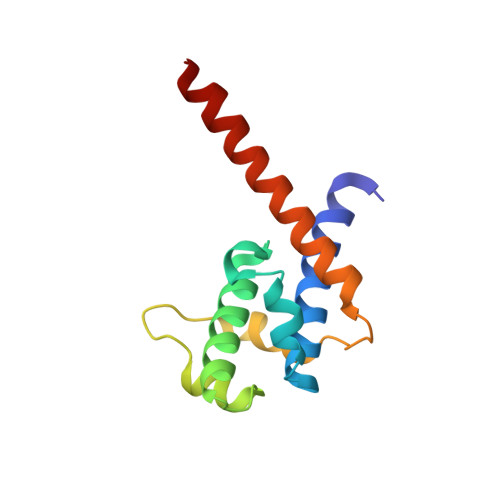
Three-dimensional structure of N-terminal domain of DnaB helicase and helicase-primase interactions in Helicobacter pylori
Kashav, T., Nitharwal, R., Abdulrehman, S.A., Gabdoulkhakov, A., Saenger, W., Dhar, S.K., Gourinath, S.(2009) PLoS One 4: e7515-e7515
- PubMed: 19841750
- DOI: https://doi.org/10.1371/journal.pone.0007515
- Primary Citation of Related Structures:
3GXV - PubMed Abstract:
Replication initiation is a crucial step in genome duplication and homohexameric DnaB helicase plays a central role in the replication initiation process by unwinding the duplex DNA and interacting with several other proteins during the process of replication. N-terminal domain of DnaB is critical for helicase activity and for DnaG primase interactions. We present here the crystal structure of the N-terminal domain (NTD) of H. pylori DnaB (HpDnaB) helicase at 2.2 A resolution and compare the structural differences among helicases and correlate with the functional differences. The structural details of NTD suggest that the linker region between NTD and C-terminal helicase domain plays a vital role in accurate assembly of NTD dimers. The sequence analysis of the linker regions from several helicases reveals that they should form four helix bundles. We also report the characterization of H. pylori DnaG primase and study the helicase-primase interactions, where HpDnaG primase stimulates DNA unwinding activity of HpDnaB suggesting presence of helicase-primase cohort at the replication fork. The protein-protein interaction study of C-terminal domain of primase and different deletion constructs of helicase suggests that linker is essential for proper conformation of NTD to interact strongly with HpDnaG. The surface charge distribution on the primase binding surface of NTDs of various helicases suggests that DnaB-DnaG interaction and stability of the complex is most probably charge dependent. Structure of the linker and helicase-primase interactions indicate that HpDnaB differs greatly from E.coli DnaB despite both belong to gram negative bacteria.
- School of Life Sciences, Jawaharlal Nehru University, New Delhi, India.
Organizational Affiliation: