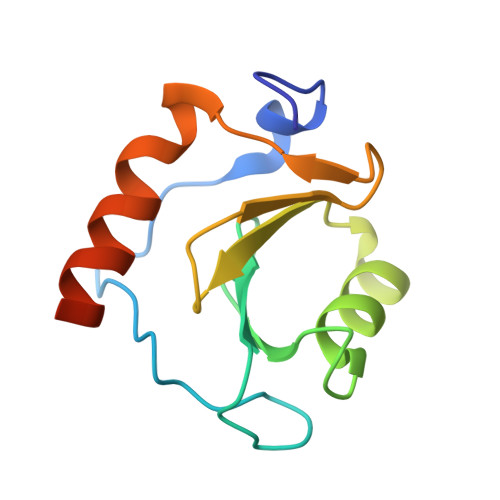
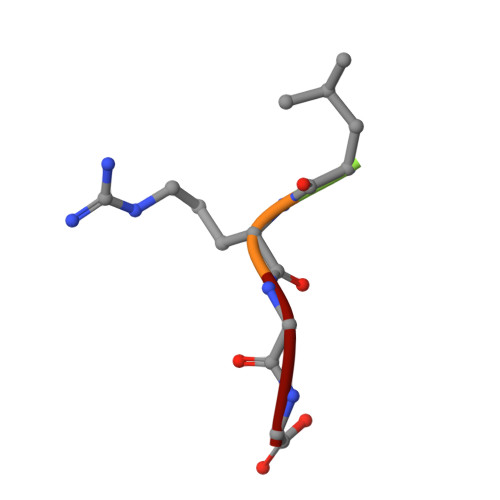
Crystal structure of human HDAC6 zinc finger domain and ubiquitin C-terminal peptide RLRGG
Dong, A., Ravichandran, M., Loppnau, P., Li, Y., MacKenzie, F., Kozieradzki, I., Edwards, A.M., Arrowsmith, C.H., Weigelt, J., Bountra, C., Bochkarev, A., Dhe-Paganon, S., Min, J., Ouyang, H.To be published.