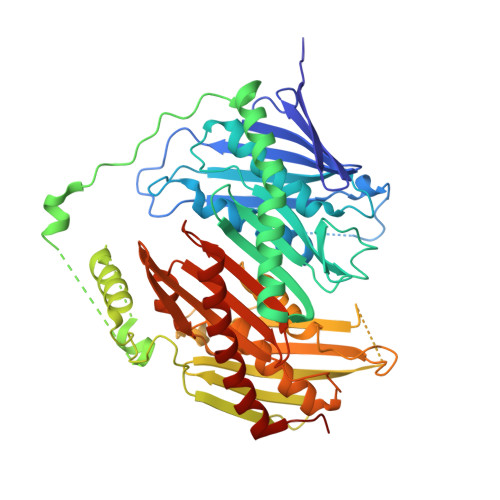
Crystal structure of Escherichia coli polynucleotide phosphorylase core bound to RNase E, RNA and manganese: implications for catalytic mechanism and RNA degradosome assembly.
Nurmohamed, S., Vaidialingam, B., Callaghan, A.J., Luisi, B.F.(2009) J Mol Biology 389: 17-33
- PubMed: 19327365
- DOI: https://doi.org/10.1016/j.jmb.2009.03.051
- Primary Citation of Related Structures:
3GCM, 3GLL, 3GME, 3H1C - PubMed Abstract:
Polynucleotide phosphorylase (PNPase) is a processive exoribonuclease that contributes to messenger RNA turnover and quality control of ribosomal RNA precursors in many bacterial species. In Escherichia coli, a proportion of the PNPase is recruited into a multi-enzyme assembly, known as the RNA degradosome, through an interaction with the scaffolding domain of the endoribonuclease RNase E. Here, we report crystal structures of E. coli PNPase complexed with the recognition site from RNase E and with manganese in the presence or in the absence of modified RNA. The homotrimeric PNPase engages RNase E on the periphery of its ring-like architecture through a pseudo-continuous anti-parallel beta-sheet. A similar interaction pattern occurs in the structurally homologous human exosome between the Rrp45 and Rrp46 subunits. At the centre of the PNPase ring is a tapered channel with an adjustable aperture where RNA bases stack on phenylalanine side chains and trigger structural changes that propagate to the active sites. Manganese can substitute for magnesium as an essential co-factor for PNPase catalysis, and our crystal structure of the enzyme in complex with manganese suggests how the metal is positioned to stabilise the transition state. We discuss the implications of these structural observations for the catalytic mechanism of PNPase, its processive mode of action, and its assembly into the RNA degradosome.
- Department of Biochemistry, University of Cambridge, UK.
Organizational Affiliation: