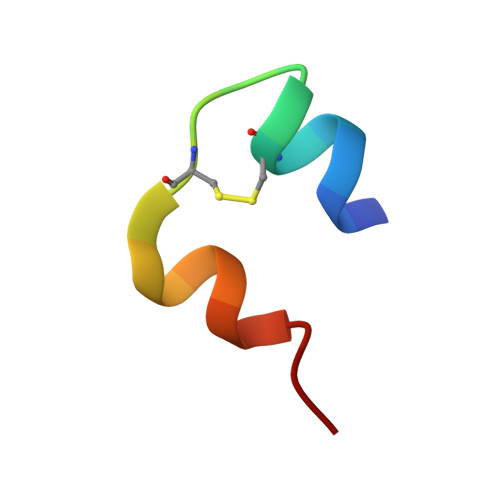
Crystal structure of a "nonfoldable" insulin: IMPAIRED FOLDING EFFICIENCY DESPITE NATIVE ACTIVITY.
Liu, M., Wan, Z.L., Chu, Y.C., Aladdin, H., Klaproth, B., Choquette, M., Hua, Q.X., Mackin, R.B., Rao, J.S., De Meyts, P., Katsoyannis, P.G., Arvan, P., Weiss, M.A.(2009) J Biological Chem 284: 35259-35272
- PubMed: 19850922
- DOI: https://doi.org/10.1074/jbc.M109.046888
- Primary Citation of Related Structures:
3GKY - PubMed Abstract:
Protein evolution is constrained by folding efficiency ("foldability") and the implicit threat of toxic misfolding. A model is provided by proinsulin, whose misfolding is associated with beta-cell dysfunction and diabetes mellitus. An insulin analogue containing a subtle core substitution (Leu(A16) --> Val) is biologically active, and its crystal structure recapitulates that of the wild-type protein. As a seeming paradox, however, Val(A16) blocks both insulin chain combination and the in vitro refolding of proinsulin. Disulfide pairing in mammalian cell culture is likewise inefficient, leading to misfolding, endoplasmic reticular stress, and proteosome-mediated degradation. Val(A16) destabilizes the native state and so presumably perturbs a partial fold that directs initial disulfide pairing. Substitutions elsewhere in the core similarly destabilize the native state but, unlike Val(A16), preserve folding efficiency. We propose that Leu(A16) stabilizes nonlocal interactions between nascent alpha-helices in the A- and B-domains to facilitate initial pairing of Cys(A20) and Cys(B19), thus surmounting their wide separation in sequence. Although Val(A16) is likely to destabilize this proto-core, its structural effects are mitigated once folding is achieved. Classical studies of insulin chain combination in vitro have illuminated the impact of off-pathway reactions on the efficiency of native disulfide pairing. The capability of a polypeptide sequence to fold within the endoplasmic reticulum may likewise be influenced by kinetic or thermodynamic partitioning among on- and off-pathway disulfide intermediates. The properties of [Val(A16)]insulin and [Val(A16)]proinsulin demonstrate that essential contributions of conserved residues to folding may be inapparent once the native state is achieved.
- Division of Metabolism, Endocrinology and Diabetes, University of Michigan Medical Center, Ann Arbor, Michigan 48109, USA.
Organizational Affiliation: