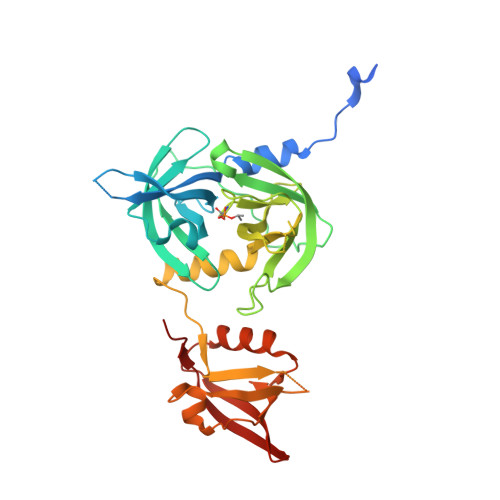
OMP peptides activate the DegS stress-sensor protease by a relief of inhibition mechanism.
Sohn, J., Grant, R.A., Sauer, R.T.(2009) Structure 17: 1411-1421
- PubMed: 19836340
- DOI: https://doi.org/10.1016/j.str.2009.07.017
- Primary Citation of Related Structures:
3GCO, 3GDS, 3GDU, 3GDV - PubMed Abstract:
In the E. coli periplasm, C-terminal peptides of misfolded outer-membrane porins (OMPs) bind to the PDZ domains of the trimeric DegS protease, triggering cleavage of a transmembrane regulator and transcriptional activation of stress genes. We show that an active-site DegS mutation partially bypasses the requirement for peptide activation and acts synergistically with mutations that disrupt contacts between the protease and PDZ domains. Biochemical results support an allosteric model, in which these mutations, active-site modification, and peptide/substrate binding act in concert to stabilize proteolytically active DegS. Cocrystal structures of DegS in complex with different OMP peptides reveal activation of the protease domain with varied conformations of the PDZ domain and without specific contacts from the bound OMP peptide. Taken together, these results indicate that the binding of OMP peptides activates proteolysis principally by relieving inhibitory contacts between the PDZ domain and the protease domain of DegS.
- Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Organizational Affiliation: