Structure of Caulobacter crescentus ClpS in complex with various peptides
Roman-Hernandez, G., Grant, R.A., Sauer, R.T., Baker, T.A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ATP-dependent Clp protease adapter protein clpS | 85 | Caulobacter vibrioides | Mutation(s): 1 Gene Names: CC_2467, clpS | 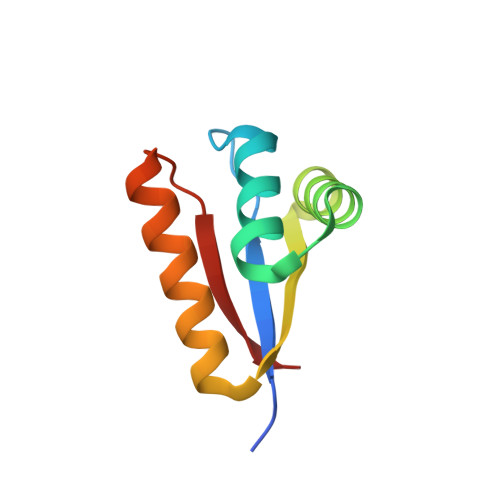 | |
UniProt | |||||
Find proteins for Q9A5I0 (Caulobacter vibrioides (strain ATCC 19089 / CIP 103742 / CB 15)) Explore Q9A5I0 Go to UniProtKB: Q9A5I0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9A5I0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Peptide (NLE)LFVQRDSKE | C [auth D] | 10 | N/A | Mutation(s): 0 | 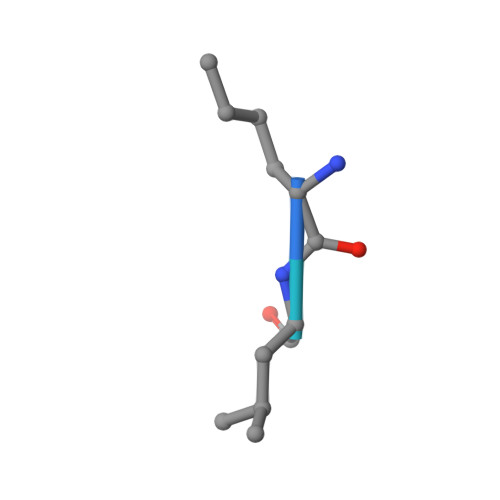 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MG Query on MG | D [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| NLE Query on NLE | C [auth D] | L-PEPTIDE LINKING | C6 H13 N O2 |  | LEU |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 33.665 | α = 90 |
| b = 54.049 | β = 110.44 |
| c = 44.615 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data collection |
| HKL-2000 | data reduction |
| PHASER | phasing |