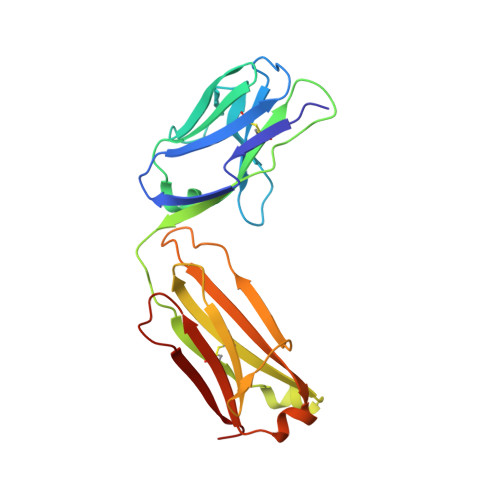
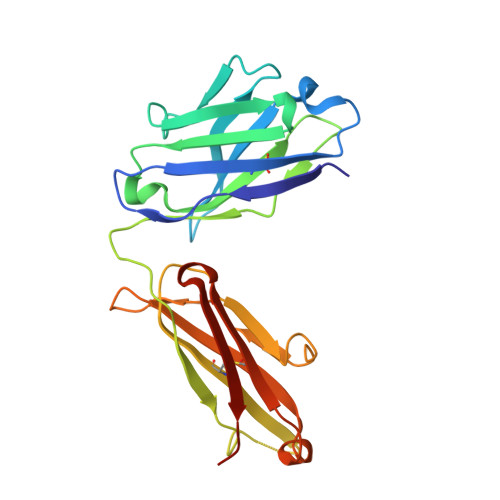
Characterization of IgG1 conformation and conformational dynamics by hydrogen/deuterium exchange mass spectrometry.
Houde, D., Arndt, J., Domeier, W., Berkowitz, S., Engen, J.R.(2009) Anal Chem 81: 2644-2651
- PubMed: 19265386
- DOI: https://doi.org/10.1021/ac802575y
- Primary Citation of Related Structures:
3FZU - PubMed Abstract:
Protein function is dictated by protein conformation. For the protein biopharmaceutical industry, therefore, it is important to have analytical tools that can detect changes in protein conformation rapidly, accurately, and with high sensitivity. In this paper we show that hydrogen/deuterium exchange mass spectrometry (H/DX-MS) can play an important role in fulfilling this need within the industry. H/DX-MS was used to assess both global and local conformational behavior of a recombinant monoclonal IgG1 antibody, a major class of biopharmaceuticals. Analysis of exchange into the intact, glycosylated IgG1 (and the Fab and Fc regions thereof) showed that the molecule was folded, highly stable, and highly amenable to analysis by this method using less than a nanomole of material. With improved chromatographic methods, peptide identification algorithms and data-processing steps, the analysis of deuterium levels in peptic peptides produced after labeling was accomplished in 1-2 days. On the basis of peptic peptide data, exchange was localized to specific regions of the antibody. Changes to IgG1 conformation as a result of deglycosylation were determined by comparing exchange into the glycosylated and deglycosylated forms of the antibody. Two regions of the IgG1 (residues 236-253 and 292-308) were found to have altered exchange properties upon deglycosylation. These results are consistent with previous findings concerning the role of glycosylation in the interaction of IgG1 with Fc receptors. Moreover, the data clearly illustrate how H/DX-MS can provide important characterization information on the higher order structure of antibodies and conformational changes that these molecules may experience upon modification.
- Biogen Idec, Inc., Cambridge Massachusetts 02142, USA. damian.houde@biogenidec.com
Organizational Affiliation: