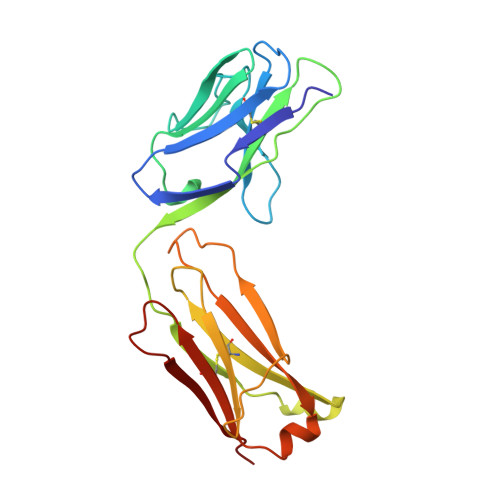
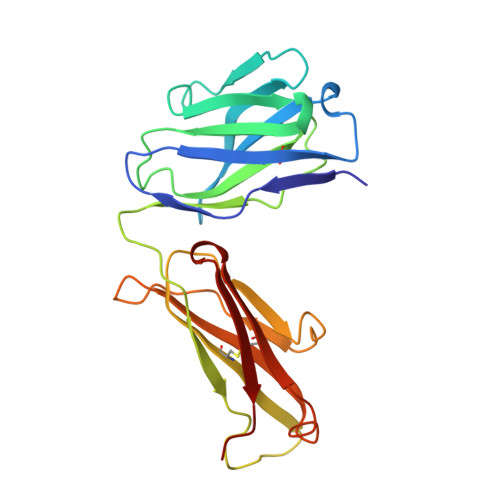
Structural and kinetic evidence for strain in biological catalysis.
Romesberg, F.E., Santarsiero, B.D., Spiller, B., Yin, J., Barnes, D., Schultz, P.G., Stevens, R.C.(1998) Biochemistry 37: 14404-14409
- PubMed: 9772166
- DOI: https://doi.org/10.1021/bi981578c
- Primary Citation of Related Structures:
3FCT - PubMed Abstract:
A classic hypothesis for enzyme catalysis is the induction of strain in the substrate. This notion was first expressed by Haldane with the lock and key analogy-"the key does not fit the lock perfectly but exercises a certain strain on it" (1). This mechanism has often been invoked to explain the catalytic efficiency of enzymes but has been difficult to establish conclusively (2-7). Here we describe X-ray crystallographic and mutational studies of an antibody metal chelatase which strongly support the notion that this antibody catalyzes metal ion insertion into the porphyrin ring by inducing strain. Analysis of the germline precursor suggests that this strain mechanism arose during the process of affinity maturation in response to a conformationally distorted N-alkylmesoporphyrin.
- Howard Hughes Medical Institute, Department of Chemistry, University of California, Berkeley 94720, USA.
Organizational Affiliation: