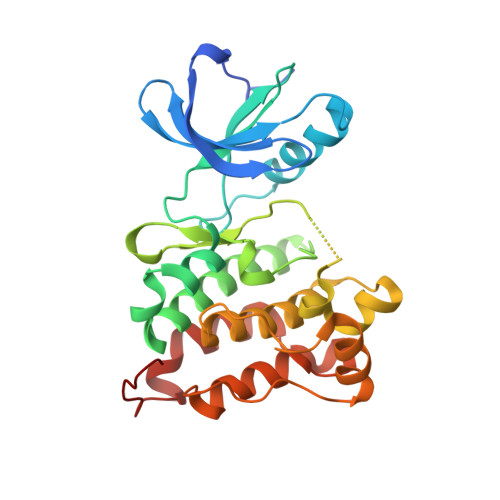
A new screening assay for allosteric inhibitors of cSrc
Simard, J.R., Kluter, S., Grutter, C., Getlik, M., Rabiller, M., Rode, H.B., Rauh, D.(2009) Nat Chem Biol 5: 394-396
- PubMed: 19396179
- DOI: https://doi.org/10.1038/nchembio.162
- Primary Citation of Related Structures:
3F3T, 3F3U - PubMed Abstract:
Targeting kinases outside the highly conserved ATP pocket is thought to be a promising strategy for overcoming bottlenecks in kinase inhibitor research, such as limited selectivity and drug resistance. Here we report the development and application of a direct binding assay to detect small molecules that stabilize the inactive conformation of the tyrosine kinase cSrc. Protein X-ray crystallography validated the assay results and confirmed an exclusively allosteric binding mode.
- Chemical Genomics Centre of the Max Planck Society, Dortmund, Germany.
Organizational Affiliation: