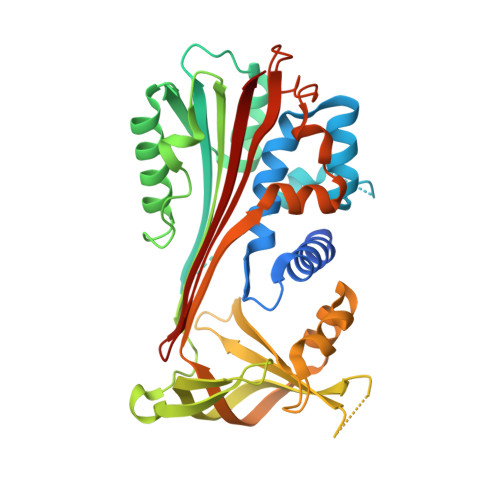
Human neuroserpin: structure and time-dependent inhibition
Ricagno, S., Caccia, S., Sorrentino, G., Antonini, G., Bolognesi, M.(2009) J Mol Biology 388: 109-121
- PubMed: 19265707
- DOI: https://doi.org/10.1016/j.jmb.2009.02.056
- Primary Citation of Related Structures:
3F02, 3F5N - PubMed Abstract:
Human neuroserpin (hNS) is a protein serine protease inhibitor expressed mainly in the nervous system, where it plays key roles in neural development and plasticity by primarily targeting tissue plasminogen activator (tPA). Four hNS mutations are associated to a form of autosomal dominant dementia, known as familial encephalopathy with neuroserpin inclusion bodies. The medical interest in and the lack of structural information on hNS prompted us to study the crystal structure of native and cleaved hNS, reported here at 3.15 and 1.85 A resolution, respectively. In the light of the three-dimensional structures, we focus on the hNS reactive centre loop in its intact and cleaved conformations relative to the current serpin polymerization models and discuss the protein sites hosting neurodegenerative mutations. On the basis of homologous serpin structures, we suggest the location of a protein surface site that may stabilize the hNS native (metastable) form. In parallel, we present the results of kinetic studies on hNS inhibition of tPA. Our data analysis stresses the instability of the hNS-tPA complex with a dissociation half-life of minutes compared to a half-life of weeks observed for other serpin-cognate protease complexes.
- Department of Biomolecular Sciences and Biotechnology, CNR-INFM and CIMAINA, University of Milano, Via Celoria 26, 20133 Milan, Italy.
Organizational Affiliation: