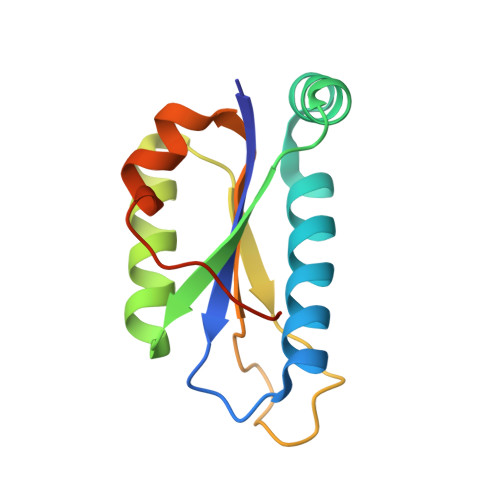
Crystal structure of the peptidyl-tRNA hydrolase AF2095 from Archaeglobus fulgidis. Northeast Structural Genomics Consortium target GR4
Forouhar, F., Su, M., Seetharaman, J., Conover, K., Janjua, H., Xiao, R., Cunningham, K., Ma, L.-C., Cooper, B., Baran, M.C., Liu, J., Acton, T.B., Montelione, G.T., Tong, L., Hunt, J.F.To be published.