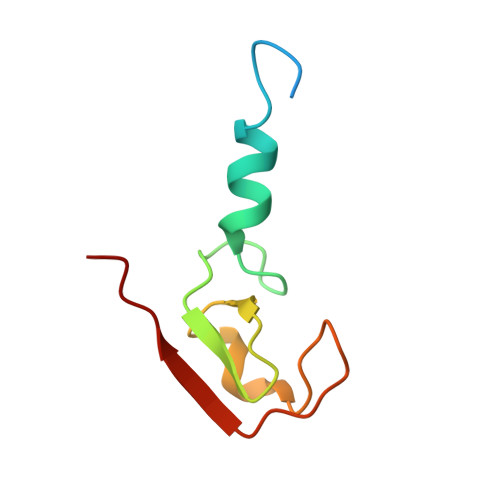
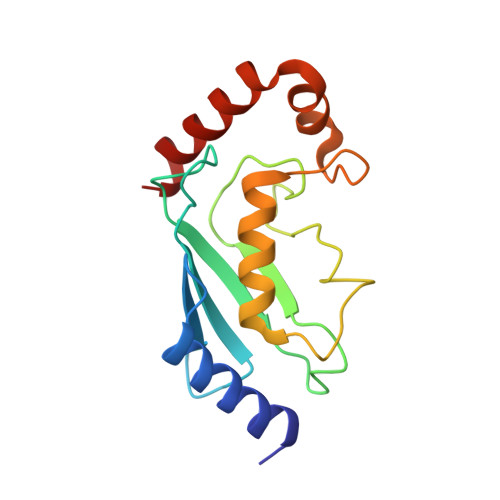
Structures of the cIAP2 RING Domain Reveal Conformational Changes Associated with Ubiquitin-conjugating Enzyme (E2) Recruitment.
Mace, P.D., Linke, K., Feltham, R., Schumacher, F.R., Smith, C.A., Vaux, D.L., Silke, J., Day, C.L.(2008) J Biological Chem 283: 31633-31640
- PubMed: 18784070
- DOI: https://doi.org/10.1074/jbc.M804753200
- Primary Citation of Related Structures:
3EB5, 3EB6 - PubMed Abstract:
Inhibitor of apoptosis (IAP) proteins are key negative regulators of cell death that are highly expressed in many cancers. Cell death caused by antagonists that bind to IAP proteins is associated with their ubiquitylation and degradation. The RING domain at the C terminus of IAP proteins is pivotal. Here we report the crystal structures of the cIAP2 RING domain homodimer alone, and bound to the ubiquitin-conjugating (E2) enzyme UbcH5b. These structures show that small changes in the RING domain accompany E2 binding. By mutating residues at the E2-binding surface, we show that autoubiquitylation is required for regulation of IAP abundance. Dimer formation is also critical, and mutation of a single C-terminal residue abrogated dimer formation and E3 ligase activity was diminished. We further demonstrate that disruption of E2 binding, or dimerization, stabilizes IAP proteins against IAP antagonists in vivo.
- Biochemistry Department, University of Otago, Dunedin 9054, New Zealand.
Organizational Affiliation: