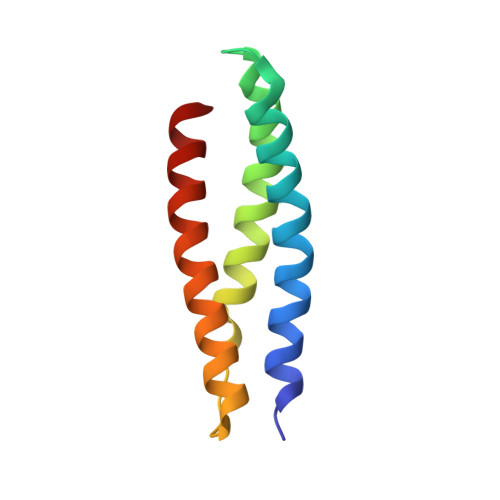
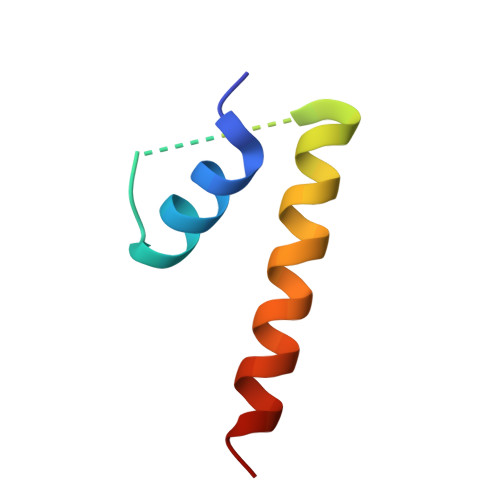
Structural basis for midbody targeting of spastin by the ESCRT-III protein CHMP1B.
Yang, D., Rismanchi, N., Renvoise, B., Lippincott-Schwartz, J., Blackstone, C., Hurley, J.H.(2008) Nat Struct Mol Biol 15: 1278-1286
- PubMed: 18997780
- DOI: https://doi.org/10.1038/nsmb.1512
- Primary Citation of Related Structures:
3EAB - PubMed Abstract:
The endosomal sorting complex required for transport (ESCRT) machinery, including ESCRT-III, localizes to the midbody and participates in the membrane-abscission step of cytokinesis. The ESCRT-III protein charged multivesicular body protein 1B (CHMP1B) is required for recruitment of the MIT domain-containing protein spastin, a microtubule-severing enzyme, to the midbody. The 2.5-A structure of the C-terminal tail of CHMP1B with the MIT domain of spastin reveals a specific, high-affinity complex involving a noncanonical binding site between the first and third helices of the MIT domain. The structural interface is twice as large as that of the MIT domain of the VPS4-CHMP complex, consistent with the high affinity of the interaction. A series of unique hydrogen-bonding interactions and close packing of small side chains discriminate against the other ten human ESCRT-III subunits. Point mutants in the CHMP1B binding site of spastin block recruitment of spastin to the midbody and impair cytokinesis.
- Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, US Department of Health and Human Services, Bethesda, Maryland 20892, USA.
Organizational Affiliation: