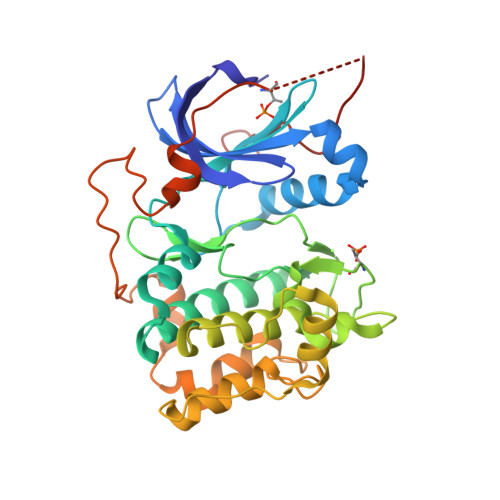
Aminofurazans as potent inhibitors of AKT kinase
Rouse, M.B., Seefeld, M.A., Leber, J.D., McNulty, K.C., Sun, L., Miller, W.H., Zhang, S., Minthorn, E.A., Concha, N.O., Choudhry, A.E., Schaber, M.D., Heerding, D.A.(2009) Bioorg Med Chem Lett 19: 1508-1511
- PubMed: 19179070
- DOI: https://doi.org/10.1016/j.bmcl.2009.01.002
- Primary Citation of Related Structures:
3E87, 3E88, 3E8C, 3E8D, 3E8E - PubMed Abstract:
AKT inhibitors containing an imidazopyridine aminofurazan scaffold have been optimized. We have previously disclosed identification of the AKT inhibitor GSK690693, which has been evaluated in clinical trials in cancer patients. Herein we describe recent efforts focusing on investigating a distinct region of this scaffold that have afforded compounds (30 and 32) with comparable activity profiles to that of GSK690693.
- Oncology Chemistry, GlaxoSmithKline, Collegeville, PA 19426, USA.
Organizational Affiliation: