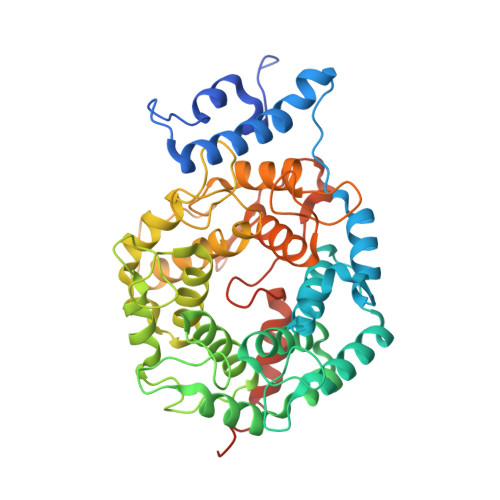
Structural basis for binding and selectivity of antimalarial and anticancer ethylenediamine inhibitors to protein farnesyltransferase.
Hast, M.A., Fletcher, S., Cummings, C.G., Pusateri, E.E., Blaskovich, M.A., Rivas, K., Gelb, M.H., Van Voorhis, W.C., Sebti, S.M., Hamilton, A.D., Beese, L.S.(2009) Chem Biol 16: 181-192
- PubMed: 19246009
- DOI: https://doi.org/10.1016/j.chembiol.2009.01.014
- Primary Citation of Related Structures:
3E30, 3E32, 3E33, 3E34, 3E37 - PubMed Abstract:
Protein farnesyltransferase (FTase) catalyzes an essential posttranslational lipid modification of more than 60 proteins involved in intracellular signal transduction networks. FTase inhibitors have emerged as a significant target for development of anticancer therapeutics and, more recently, for the treatment of parasitic diseases caused by protozoan pathogens, including malaria (Plasmodium falciparum). We present the X-ray crystallographic structures of complexes of mammalian FTase with five inhibitors based on an ethylenediamine scaffold, two of which exhibit over 1000-fold selective inhibition of P. falciparum FTase. These structures reveal the dominant determinants in both the inhibitor and enzyme that control binding and selectivity. Comparison to a homology model constructed for the P. falciparum FTase suggests opportunities for further improving selectivity of a new generation of antimalarial inhibitors.
- Department of Biochemistry, Duke University Medical Center, Box 3711, Durham, NC 27710, USA.
Organizational Affiliation: