Insights into the Replisome from the Structure of a Ternary Complex of the DNA Polymerase III alpha-Subunit.
Wing, R.A., Bailey, S., Steitz, T.A.(2008) J Mol Biology 382: 859-869
- PubMed: 18691598
- DOI: https://doi.org/10.1016/j.jmb.2008.07.058
- Primary Citation of Related Structures:
3E0D - PubMed Abstract:
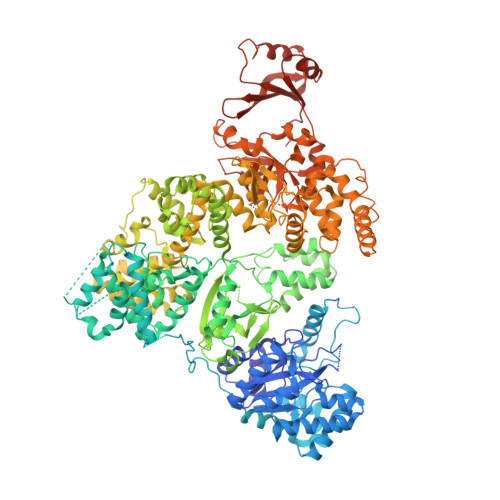
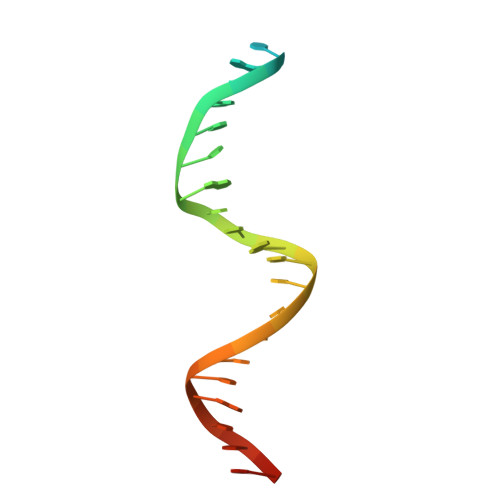
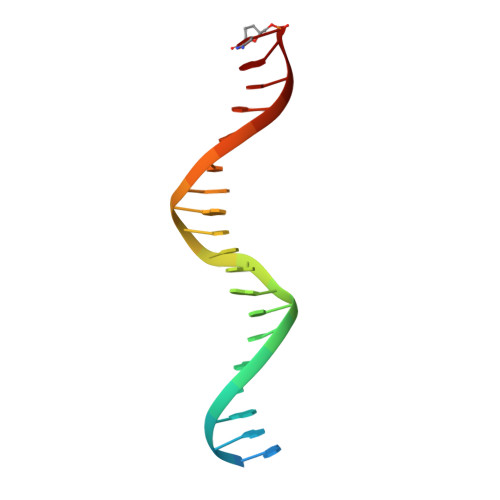
The crystal structure of the catalytic alpha-subunit of the DNA polymerase III (Pol IIIalpha) holoenzyme bound to primer-template DNA and an incoming deoxy-nucleoside 5'-triphosphate has been determined at 4.6-A resolution. The polymerase interacts with the sugar-phosphate backbone of the DNA across its minor groove, which is made possible by significant movements of the thumb, finger, and beta-binding domains relative to their orientations in the unliganded polymerase structure. Additionally, the DNA and incoming nucleotide are bound to the active site of Pol IIIalpha nearly identically as they are in their complex with DNA polymerase beta, thereby proving that the eubacterial replicating polymerase, but not the eukaryotic replicating polymerase, is homologous to DNA polymerase beta. Finally, superimposing a recent structure of the clamp bound to DNA on this Pol IIIalpha complex with DNA places a loop of the beta-binding domain into the appropriate clamp cleft and supports a mechanism of polymerase switching.
- Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06520, USA.
Organizational Affiliation: